Tuesday Poster Session
Category: Infections and Microbiome
P5641 - A Rare Case of Hepatitis B Reactivation in a Patient Treated With Regorafenib for Gastrointestinal Stromal Tumor: A Case Report
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Hayder Alamily, MD (he/him/his)
University of Colorado Anschutz Medical Campus
Denver, CO
Presenting Author(s)
Hayder Alamily, MD1, Sana Rabeeah, MD2, Omer Najem, MD3, Ban Mashadani, MD4, Bisher Sawaf, MD5, Priyanka Prakash, MD4, Sajjad Al-Badri, MBChB6, Scott Wofford, MD7, Paula Podrazik, MD4, Maxine Seales-Kasangana, MD4, Samuel Hunter. Dunn, MD4
1University of Colorado Anschutz Medical Campus, Denver, CO; 2The University of Toledo, Toledo, OH; 3Department of internal medicine, Trinity Health Oakland, Pontiac, MI; 4Baptist Health-University of Arkansas for Medical Sciences, North Little Rock, AR; 5University of Toledo Medical Center, Toledo, OH; 6College of Medicine, University of Baghdad, Baghdad, Baghdad, Iraq; 7Baptist Health-University of Arkansas for Medical Sciences, Little Rock, AR
Introduction: Regorafenib, a multikinase inhibitor approved for advanced gastrointestinal stromal tumors (GIST), is known for its hepatotoxic potential. However, hepatitis B virus (HBV) reactivation is a rarely reported complication. We present a case of HBV reactivation leading to severe hepatic injury without liver failure in a patient receiving regorafenib, compounded by concurrent Mycobacterium avium complex (MAC) infection.
Case Description/
Methods: A 66-year-old woman with metastatic GIST and history of breast cancer presented with weight loss and cough. Imaging revealed cavitary lung lesions, and MAC was confirmed on bronchoscopy. She was started on azithromycin, ethambutol, and rifampin. Months later, she developed jaundice and elevated liver enzymes. HBV serologies showed HBsAg positivity, anti-HBe positivity, positive anti-HBs, due to prior vaccination and high HBV DNA levels. Liver biopsy confirmed severe fibrosis (F4) with active inflammation (A3), consistent with HBV reactivation and severe hepatic injury. Tenofovir was initiated but discontinued due to Fanconi syndrome; she was transitioned to entecavir. MAC therapy was held to reduce hepatic burden. Serial monitoring showed a steady decline in viral load, HBV DNA reached minimal levels by December 2024. Liver function normalized, and the patient recovered clinically. She remains on long-term entecavir with well-controlled viremia with no recurrence of HBV.
Discussion: This case highlights a rare but severe complication of regorafenib: HBV reactivation with severe hepatic injury. Tyrosine kinase inhibitors such as regorafenib may disrupt immune surveillance, leading to viral reactivation in patients with latent HBV. The coexisting MAC infection and prolonged antimicrobial therapy may have further impaired immune function. Antiviral therapy with nucleos(t)ide analogues remains the mainstay of treatment, but renal toxicity may necessitate switching agents. This case underscores the importance of routine HBV screening and prophylaxis prior to initiating immunosuppressive or targeted cancer therapies. Early identification and management of HBV reactivation are crucial to prevent life-threatening hepatic complications.
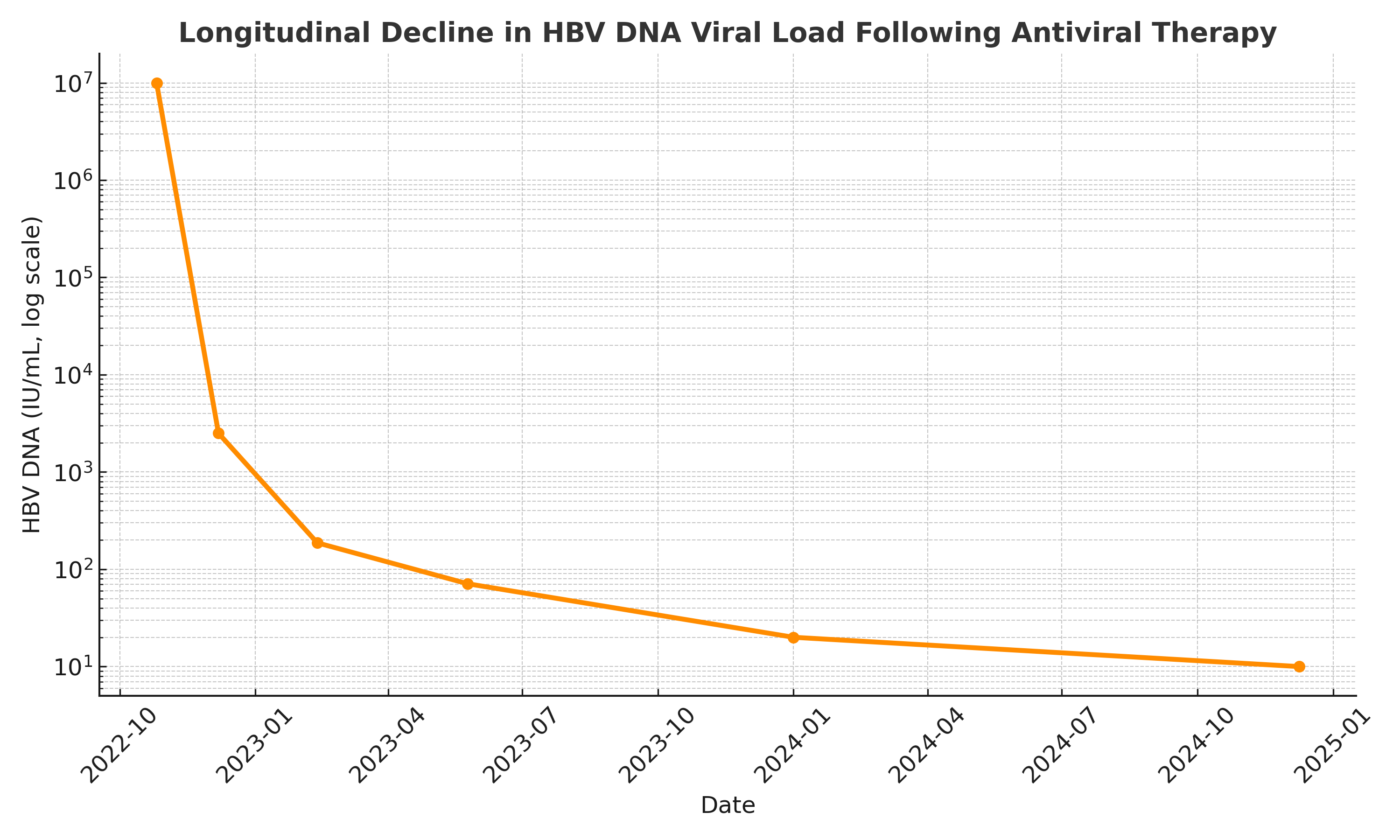
Figure: HBV Viral Load Timeline
Disclosures:
Hayder Alamily indicated no relevant financial relationships.
Sana Rabeeah indicated no relevant financial relationships.
Omer Najem indicated no relevant financial relationships.
Ban Mashadani indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Priyanka Prakash indicated no relevant financial relationships.
Sajjad Al-Badri indicated no relevant financial relationships.
Scott Wofford indicated no relevant financial relationships.
Paula Podrazik indicated no relevant financial relationships.
Maxine Seales-Kasangana indicated no relevant financial relationships.
Samuel Dunn indicated no relevant financial relationships.
Hayder Alamily, MD1, Sana Rabeeah, MD2, Omer Najem, MD3, Ban Mashadani, MD4, Bisher Sawaf, MD5, Priyanka Prakash, MD4, Sajjad Al-Badri, MBChB6, Scott Wofford, MD7, Paula Podrazik, MD4, Maxine Seales-Kasangana, MD4, Samuel Hunter. Dunn, MD4. P5641 - A Rare Case of Hepatitis B Reactivation in a Patient Treated With Regorafenib for Gastrointestinal Stromal Tumor: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Colorado Anschutz Medical Campus, Denver, CO; 2The University of Toledo, Toledo, OH; 3Department of internal medicine, Trinity Health Oakland, Pontiac, MI; 4Baptist Health-University of Arkansas for Medical Sciences, North Little Rock, AR; 5University of Toledo Medical Center, Toledo, OH; 6College of Medicine, University of Baghdad, Baghdad, Baghdad, Iraq; 7Baptist Health-University of Arkansas for Medical Sciences, Little Rock, AR
Introduction: Regorafenib, a multikinase inhibitor approved for advanced gastrointestinal stromal tumors (GIST), is known for its hepatotoxic potential. However, hepatitis B virus (HBV) reactivation is a rarely reported complication. We present a case of HBV reactivation leading to severe hepatic injury without liver failure in a patient receiving regorafenib, compounded by concurrent Mycobacterium avium complex (MAC) infection.
Case Description/
Methods: A 66-year-old woman with metastatic GIST and history of breast cancer presented with weight loss and cough. Imaging revealed cavitary lung lesions, and MAC was confirmed on bronchoscopy. She was started on azithromycin, ethambutol, and rifampin. Months later, she developed jaundice and elevated liver enzymes. HBV serologies showed HBsAg positivity, anti-HBe positivity, positive anti-HBs, due to prior vaccination and high HBV DNA levels. Liver biopsy confirmed severe fibrosis (F4) with active inflammation (A3), consistent with HBV reactivation and severe hepatic injury. Tenofovir was initiated but discontinued due to Fanconi syndrome; she was transitioned to entecavir. MAC therapy was held to reduce hepatic burden. Serial monitoring showed a steady decline in viral load, HBV DNA reached minimal levels by December 2024. Liver function normalized, and the patient recovered clinically. She remains on long-term entecavir with well-controlled viremia with no recurrence of HBV.
Discussion: This case highlights a rare but severe complication of regorafenib: HBV reactivation with severe hepatic injury. Tyrosine kinase inhibitors such as regorafenib may disrupt immune surveillance, leading to viral reactivation in patients with latent HBV. The coexisting MAC infection and prolonged antimicrobial therapy may have further impaired immune function. Antiviral therapy with nucleos(t)ide analogues remains the mainstay of treatment, but renal toxicity may necessitate switching agents. This case underscores the importance of routine HBV screening and prophylaxis prior to initiating immunosuppressive or targeted cancer therapies. Early identification and management of HBV reactivation are crucial to prevent life-threatening hepatic complications.

Figure: HBV Viral Load Timeline
Disclosures:
Hayder Alamily indicated no relevant financial relationships.
Sana Rabeeah indicated no relevant financial relationships.
Omer Najem indicated no relevant financial relationships.
Ban Mashadani indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Priyanka Prakash indicated no relevant financial relationships.
Sajjad Al-Badri indicated no relevant financial relationships.
Scott Wofford indicated no relevant financial relationships.
Paula Podrazik indicated no relevant financial relationships.
Maxine Seales-Kasangana indicated no relevant financial relationships.
Samuel Dunn indicated no relevant financial relationships.
Hayder Alamily, MD1, Sana Rabeeah, MD2, Omer Najem, MD3, Ban Mashadani, MD4, Bisher Sawaf, MD5, Priyanka Prakash, MD4, Sajjad Al-Badri, MBChB6, Scott Wofford, MD7, Paula Podrazik, MD4, Maxine Seales-Kasangana, MD4, Samuel Hunter. Dunn, MD4. P5641 - A Rare Case of Hepatitis B Reactivation in a Patient Treated With Regorafenib for Gastrointestinal Stromal Tumor: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
