Tuesday Poster Session
Category: IBD
P5472 - Comparative Efficacy and Tolerability of Janus Kinas Inhibitors for Moderate to Severe Crohn’s Disease - A Systematic Review and Bayesian Network Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Amine Rakab, MD (he/him/his)
Division of Medical Education, Weill Cornell Medicine
Doha, Ad Dawhah, Qatar
Presenting Author(s)
Hakim Wazir, MBBS1, Saniya Ishtiaq, MD2, Muhammad Maaz, MBBS3, Bisher Sawaf, MD4, Amine Rakab, MD5, Amin Abu Hejleh, MD6, Maryam Muzaffar, MD7, M. Rafiqul Islam, 8, Nimra Ehsan, MD9, Ammarah Tariq, MD2, Saba Aliha, MD10, Anchit Chauhan, 11, Nayanika Tummala, MD12
1Gajju Khan Medical College,Swabi Pakistan Medicine department, Shahmansor, Swabi, North-West Frontier, Pakistan; 2Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 3Bacha Khan Medical College, Mardan Pakistan, Mardan, North-West Frontier, Pakistan; 4The University of Toledo, Toledo, OH; 5Division of Medical Education, Weill Cornell Medicine, Doha, Ad Dawhah, Qatar; 6Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, Dubai, United Arab Emirates; 7Allama Iqbal medical college, Lahore, Punjab, Pakistan; 8Shaheed Suhrawardy Medical College and Hospital, Dhaka, Bangladesh, Dhaka, Dhaka, Bangladesh; 9Khyber medical college Peshawar, Peshawar, North-West Frontier, Pakistan; 10Multan Medical and Dental College, Multan, Punjab, Pakistan; 11Maulana Azad Medical College, New Delhi, Delhi, India; 12St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY
Introduction: Crohn’s disease, a chronic Inflammatory bowel disease, has limited treatments with variable efficacy and side effects. Janus kinase (JAK) inhibitors offer promise by targeting inflammatory cytokine pathways. This network meta-analysis systematically evaluates their comparative efficacy and safety in moderate-to-severe Crohn’s, incorporating the latest randomised clinical trials evidence.
Methods: We conducted a thorough search on PubMed, Cochrane, Embase, Scopus and ClinicalTrials.gov. Screening used Rayyan with data extracted to Excel. Analyses was done on R 4.4.3 software, including Bayesian network meta-analysis with pooled mean differences and hazard ratios with a 95% confidence interval via random/fixed-effects models based on deviance information criteria (DIC). Surface under the cumulative ranking curve (SURCA) values ranked interventions for clinical/endoscopic remission, response, and safety outcomes.
Results: A total of 12 RCTs with 25 interventions were included involving 5,129 patients.Upadacitinib 45 mg once daily OD showed significant efficacy in achieving clinical remission compared to placebo (HR:2.47; 95% CrI: 1.51–4.10), supported by the highest SUCRA value of 0.79 and a significant MD (MD: 0.907; CrI: 0.410–1.41). For endoscopic remission, Upada 45 mg OD was also superior to placebo (HR: 5.89; CrI: 2.79–13.86), though Upada 24 mg QD had a higher SUCRA value (0.87 vs 0.38). Upada 24 mg QD showed the most favorable trend in clinical response (HR: 2.18; CrI: 0.93–5.22; SUCRA: 0.74) while Upada 45 mg OD had lower effectiveness (HR: 1.25; CrI: 0.82–1.90; SUCRA: 0.30). In endoscopic response, Upada 24 mg QD ranked highest (HR: 17.89; CrI: 2.66–340.59; SUCRA: 0.85), followed by Upada 45 mg OD (HR: 6.56; CrI: 3.14–15.07; SUCRA: 0.56). Filgotinib 200 mg OD showed the best safety for total adverse events (HR: 0.93; CrI: 0.78–1.10; SUCRA: 0.74), while Upada 45 mg OD had the lowest SUCRA (0.13). For serious AE, Upada 45 mg OD had an HR of 1.08 (CrI: 0.92–1.28) but the highest SUCRA (0.71). In GI perforation, Filgotinib 200 mg OD had HR of 0.95 (CrI: 0.18–4.65; SUCRA: 0.63) compared to Upada 45 mg OD (HR: 1.40; CrI: 0.04–106.14; SUCRA: 0.46).
Discussion: Upada 45 mg OD demonstrated superior efficacy, particularly for remission outcomes, while 24 mg QD showed favorable clinical/endoscopic remission trends. Filgotinib 200 mg OD exhibited the best safety profile with fewer adverse events.
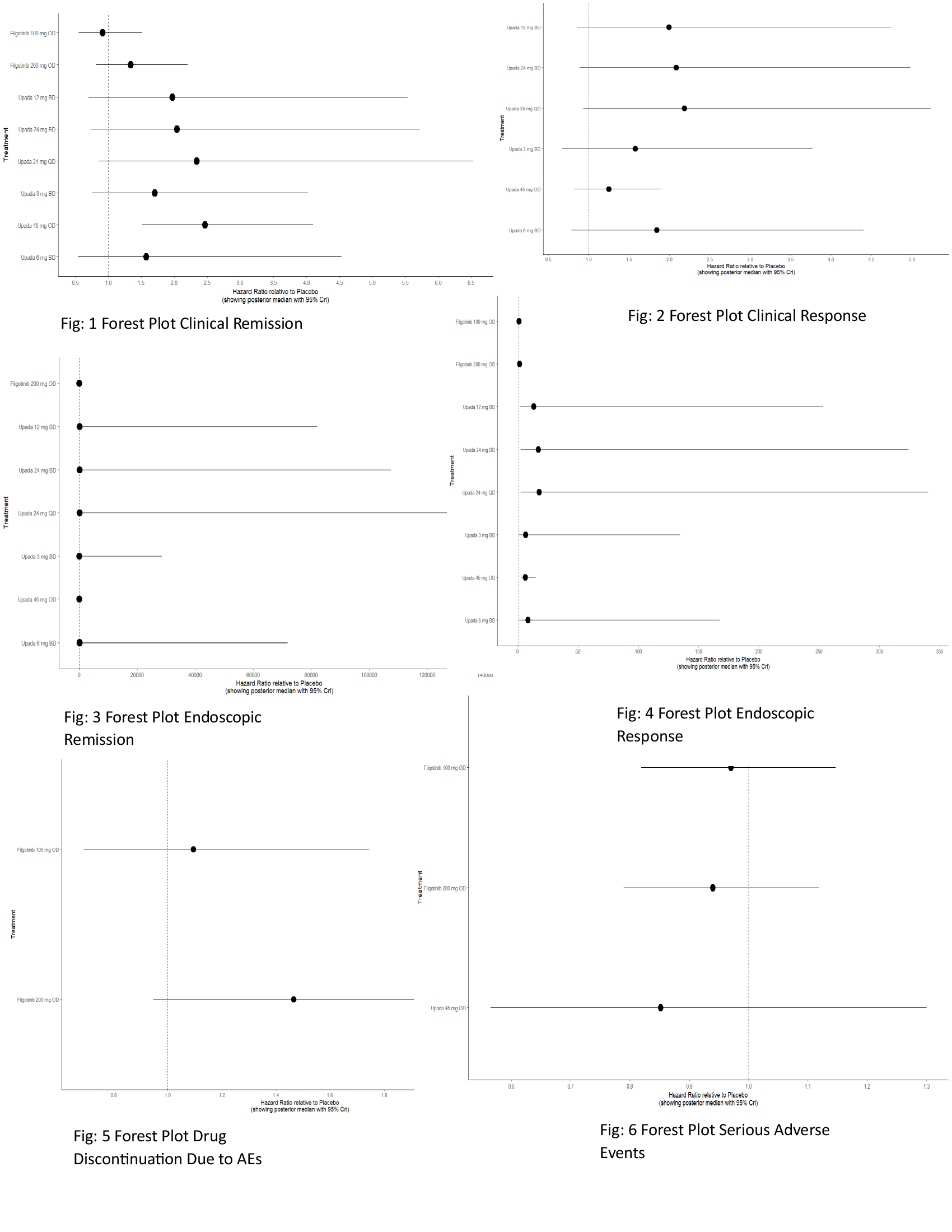
Figure: Figure 1: Forest Plot Clinical Remission
Figure 2: Forest Plot Clinical Response
Figure 3: Forest Plot Endoscopic Remission
Figure 4: Forest Plot Endoscopic Response
Figure 5: Forest Plot Drug Discontinuation Due to AEs
Figure 6: Forest Plot Serious Adverse Events
Disclosures:
Hakim Wazir indicated no relevant financial relationships.
Saniya Ishtiaq indicated no relevant financial relationships.
Muhammad Maaz indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Amine Rakab indicated no relevant financial relationships.
Amin Abu Hejleh indicated no relevant financial relationships.
Maryam Muzaffar indicated no relevant financial relationships.
M. Rafiqul Islam indicated no relevant financial relationships.
Nimra Ehsan indicated no relevant financial relationships.
Ammarah Tariq indicated no relevant financial relationships.
Saba Aliha indicated no relevant financial relationships.
Anchit Chauhan indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Hakim Wazir, MBBS1, Saniya Ishtiaq, MD2, Muhammad Maaz, MBBS3, Bisher Sawaf, MD4, Amine Rakab, MD5, Amin Abu Hejleh, MD6, Maryam Muzaffar, MD7, M. Rafiqul Islam, 8, Nimra Ehsan, MD9, Ammarah Tariq, MD2, Saba Aliha, MD10, Anchit Chauhan, 11, Nayanika Tummala, MD12. P5472 - Comparative Efficacy and Tolerability of Janus Kinas Inhibitors for Moderate to Severe Crohn’s Disease - A Systematic Review and Bayesian Network Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Gajju Khan Medical College,Swabi Pakistan Medicine department, Shahmansor, Swabi, North-West Frontier, Pakistan; 2Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 3Bacha Khan Medical College, Mardan Pakistan, Mardan, North-West Frontier, Pakistan; 4The University of Toledo, Toledo, OH; 5Division of Medical Education, Weill Cornell Medicine, Doha, Ad Dawhah, Qatar; 6Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, Dubai, United Arab Emirates; 7Allama Iqbal medical college, Lahore, Punjab, Pakistan; 8Shaheed Suhrawardy Medical College and Hospital, Dhaka, Bangladesh, Dhaka, Dhaka, Bangladesh; 9Khyber medical college Peshawar, Peshawar, North-West Frontier, Pakistan; 10Multan Medical and Dental College, Multan, Punjab, Pakistan; 11Maulana Azad Medical College, New Delhi, Delhi, India; 12St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY
Introduction: Crohn’s disease, a chronic Inflammatory bowel disease, has limited treatments with variable efficacy and side effects. Janus kinase (JAK) inhibitors offer promise by targeting inflammatory cytokine pathways. This network meta-analysis systematically evaluates their comparative efficacy and safety in moderate-to-severe Crohn’s, incorporating the latest randomised clinical trials evidence.
Methods: We conducted a thorough search on PubMed, Cochrane, Embase, Scopus and ClinicalTrials.gov. Screening used Rayyan with data extracted to Excel. Analyses was done on R 4.4.3 software, including Bayesian network meta-analysis with pooled mean differences and hazard ratios with a 95% confidence interval via random/fixed-effects models based on deviance information criteria (DIC). Surface under the cumulative ranking curve (SURCA) values ranked interventions for clinical/endoscopic remission, response, and safety outcomes.
Results: A total of 12 RCTs with 25 interventions were included involving 5,129 patients.Upadacitinib 45 mg once daily OD showed significant efficacy in achieving clinical remission compared to placebo (HR:2.47; 95% CrI: 1.51–4.10), supported by the highest SUCRA value of 0.79 and a significant MD (MD: 0.907; CrI: 0.410–1.41). For endoscopic remission, Upada 45 mg OD was also superior to placebo (HR: 5.89; CrI: 2.79–13.86), though Upada 24 mg QD had a higher SUCRA value (0.87 vs 0.38). Upada 24 mg QD showed the most favorable trend in clinical response (HR: 2.18; CrI: 0.93–5.22; SUCRA: 0.74) while Upada 45 mg OD had lower effectiveness (HR: 1.25; CrI: 0.82–1.90; SUCRA: 0.30). In endoscopic response, Upada 24 mg QD ranked highest (HR: 17.89; CrI: 2.66–340.59; SUCRA: 0.85), followed by Upada 45 mg OD (HR: 6.56; CrI: 3.14–15.07; SUCRA: 0.56). Filgotinib 200 mg OD showed the best safety for total adverse events (HR: 0.93; CrI: 0.78–1.10; SUCRA: 0.74), while Upada 45 mg OD had the lowest SUCRA (0.13). For serious AE, Upada 45 mg OD had an HR of 1.08 (CrI: 0.92–1.28) but the highest SUCRA (0.71). In GI perforation, Filgotinib 200 mg OD had HR of 0.95 (CrI: 0.18–4.65; SUCRA: 0.63) compared to Upada 45 mg OD (HR: 1.40; CrI: 0.04–106.14; SUCRA: 0.46).
Discussion: Upada 45 mg OD demonstrated superior efficacy, particularly for remission outcomes, while 24 mg QD showed favorable clinical/endoscopic remission trends. Filgotinib 200 mg OD exhibited the best safety profile with fewer adverse events.

Figure: Figure 1: Forest Plot Clinical Remission
Figure 2: Forest Plot Clinical Response
Figure 3: Forest Plot Endoscopic Remission
Figure 4: Forest Plot Endoscopic Response
Figure 5: Forest Plot Drug Discontinuation Due to AEs
Figure 6: Forest Plot Serious Adverse Events
Disclosures:
Hakim Wazir indicated no relevant financial relationships.
Saniya Ishtiaq indicated no relevant financial relationships.
Muhammad Maaz indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Amine Rakab indicated no relevant financial relationships.
Amin Abu Hejleh indicated no relevant financial relationships.
Maryam Muzaffar indicated no relevant financial relationships.
M. Rafiqul Islam indicated no relevant financial relationships.
Nimra Ehsan indicated no relevant financial relationships.
Ammarah Tariq indicated no relevant financial relationships.
Saba Aliha indicated no relevant financial relationships.
Anchit Chauhan indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Hakim Wazir, MBBS1, Saniya Ishtiaq, MD2, Muhammad Maaz, MBBS3, Bisher Sawaf, MD4, Amine Rakab, MD5, Amin Abu Hejleh, MD6, Maryam Muzaffar, MD7, M. Rafiqul Islam, 8, Nimra Ehsan, MD9, Ammarah Tariq, MD2, Saba Aliha, MD10, Anchit Chauhan, 11, Nayanika Tummala, MD12. P5472 - Comparative Efficacy and Tolerability of Janus Kinas Inhibitors for Moderate to Severe Crohn’s Disease - A Systematic Review and Bayesian Network Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
