Tuesday Poster Session
Category: IBD
P5459 - Advanced Therapies in IBD and Risk of Active Tuberculosis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Karan Sachdeva, MBBS, MPH
SUNY Upstate Medical University
Syracuse, NY
Presenting Author(s)
Karan Sachdeva, MBBS, MPH, Ahmad Nawaz, MBBS, Idan Goren, MD
SUNY Upstate Medical University, Syracuse, NY
Introduction: Advanced therapies (ATs), including tumor necrosis factor (TNF) inhibitors and non-TNF ATs—such as Janus kinase (JAK) inhibitors, integrin receptor antagonists, interleukin (IL) inhibitors, and sphingosine-1-phosphate (S1P) receptor modulators—are widely used in the treatment of inflammatory bowel disease (IBD). In the U.S., baseline and annual tuberculosis (TB) screening is universally required for insurance coverage of biologic and small molecule therapies, regardless of the actual risk. We aimed to evaluate the risk of active TB in IBD patients, stratified by AT type.
Methods: We conducted a retrospective cohort study using TriNetX, a federated global research network comprising de-identified electronic health records from U.S. healthcare organizations. IBD patients were identified between January 2000 and January 2024. Patients were categorized into three cohorts: (1) those treated with TNF inhibitors, (2) those receiving non-TNF AT (JAK inhibitors, integrin receptor antagonists, IL inhibitors, or S1P receptor modulators), and (3) those naïve to AT. The primary outcome was active TB diagnosis (ICD-10 codes A15-19). Propensity score matching (1:1) was applied to adjust for demographics (age, sex, race) and clinical confounders (HIV infection, chronic kidney disease, nicotine dependence, corticosteroid use, chemotherapy, and autoimmune diseases). Adjusted odd’s ratios (aORs) with 95% confidence intervals (CIs) were calculated.
Results: Among 102,019 IBD patients treated with AT and followed for a total of 357,066.5 patient-years, 280 were diagnosed with active TB compared to 90 cases in patients naïve to AT (aOR=2.3; 95% CI: 1.8–2.9). Patients receiving TNF inhibitors (n = 63,560) had a significantly increased risk of active TB compared to AT–naïve patients (126 vs. 65 cases; aOR=1.9; 95% CI: 1.4–2.6). In contrast, patients on non-TNF AT (n = 31,369) did not demonstrate an increased active TB risk relative to their AT–naïve counterparts (24 vs. 35 cases; aOR=0.69; 95% CI: 0.4–1.1). In a direct comparison between TNF and non-TNF AT treated patients (n = 20,104 each), TNF inhibitor use was associated with a threefold higher risk of active TB (aOR=3.0; 95% CI: 1.6–5.4).
Discussion: In this large, real-world cohort of IBD patients, TNF inhibitors were associated with a significantly increased risk of active TB, whereas non-TNF advanced therapies were not. These findings support a risk-stratified approach to TB screening based on the specific class of advanced therapy.
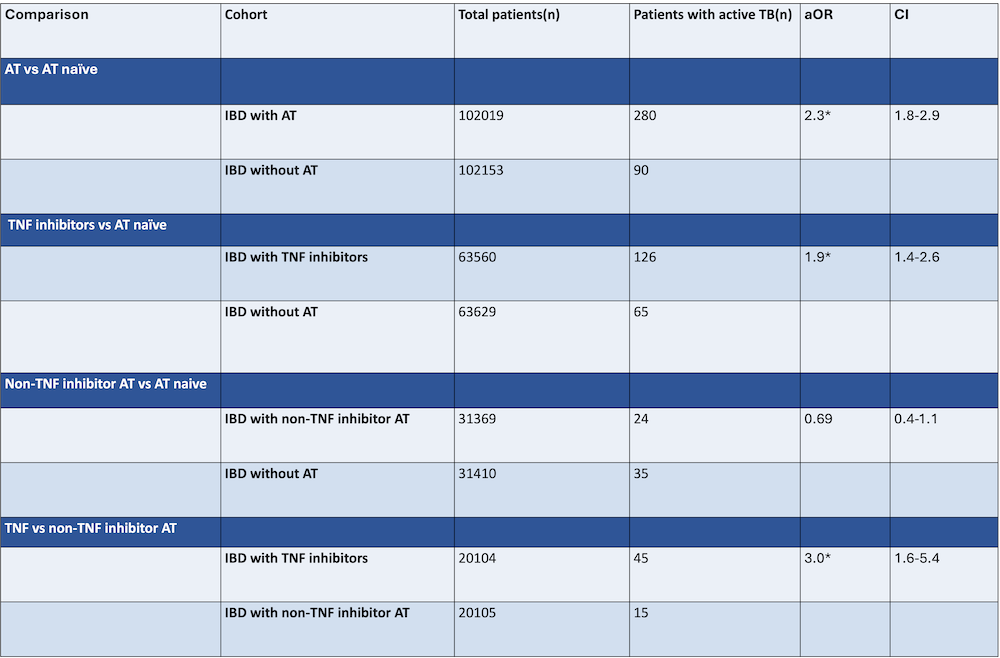
Figure: Table 1: Total number of patients after propensity score matching. aOR, adjusted odd’s ratio; CI, confidence interval; AT, advanced therapies; TNF, tumor necrosis factor. *statistically significant
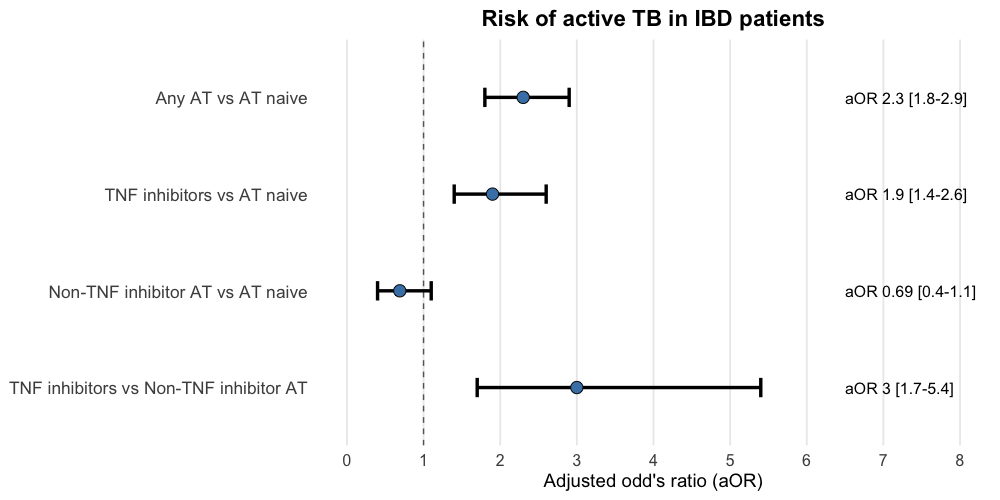
Figure: Figure 1. Advanced therapies and risk of active tuberculosis in IBD patients. AT, advanced therapies; TNF, tumor necrosis factor
Disclosures:
Karan Sachdeva indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Idan Goren indicated no relevant financial relationships.
Karan Sachdeva, MBBS, MPH, Ahmad Nawaz, MBBS, Idan Goren, MD. P5459 - Advanced Therapies in IBD and Risk of Active Tuberculosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
SUNY Upstate Medical University, Syracuse, NY
Introduction: Advanced therapies (ATs), including tumor necrosis factor (TNF) inhibitors and non-TNF ATs—such as Janus kinase (JAK) inhibitors, integrin receptor antagonists, interleukin (IL) inhibitors, and sphingosine-1-phosphate (S1P) receptor modulators—are widely used in the treatment of inflammatory bowel disease (IBD). In the U.S., baseline and annual tuberculosis (TB) screening is universally required for insurance coverage of biologic and small molecule therapies, regardless of the actual risk. We aimed to evaluate the risk of active TB in IBD patients, stratified by AT type.
Methods: We conducted a retrospective cohort study using TriNetX, a federated global research network comprising de-identified electronic health records from U.S. healthcare organizations. IBD patients were identified between January 2000 and January 2024. Patients were categorized into three cohorts: (1) those treated with TNF inhibitors, (2) those receiving non-TNF AT (JAK inhibitors, integrin receptor antagonists, IL inhibitors, or S1P receptor modulators), and (3) those naïve to AT. The primary outcome was active TB diagnosis (ICD-10 codes A15-19). Propensity score matching (1:1) was applied to adjust for demographics (age, sex, race) and clinical confounders (HIV infection, chronic kidney disease, nicotine dependence, corticosteroid use, chemotherapy, and autoimmune diseases). Adjusted odd’s ratios (aORs) with 95% confidence intervals (CIs) were calculated.
Results: Among 102,019 IBD patients treated with AT and followed for a total of 357,066.5 patient-years, 280 were diagnosed with active TB compared to 90 cases in patients naïve to AT (aOR=2.3; 95% CI: 1.8–2.9). Patients receiving TNF inhibitors (n = 63,560) had a significantly increased risk of active TB compared to AT–naïve patients (126 vs. 65 cases; aOR=1.9; 95% CI: 1.4–2.6). In contrast, patients on non-TNF AT (n = 31,369) did not demonstrate an increased active TB risk relative to their AT–naïve counterparts (24 vs. 35 cases; aOR=0.69; 95% CI: 0.4–1.1). In a direct comparison between TNF and non-TNF AT treated patients (n = 20,104 each), TNF inhibitor use was associated with a threefold higher risk of active TB (aOR=3.0; 95% CI: 1.6–5.4).
Discussion: In this large, real-world cohort of IBD patients, TNF inhibitors were associated with a significantly increased risk of active TB, whereas non-TNF advanced therapies were not. These findings support a risk-stratified approach to TB screening based on the specific class of advanced therapy.

Figure: Table 1: Total number of patients after propensity score matching. aOR, adjusted odd’s ratio; CI, confidence interval; AT, advanced therapies; TNF, tumor necrosis factor. *statistically significant

Figure: Figure 1. Advanced therapies and risk of active tuberculosis in IBD patients. AT, advanced therapies; TNF, tumor necrosis factor
Disclosures:
Karan Sachdeva indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Idan Goren indicated no relevant financial relationships.
Karan Sachdeva, MBBS, MPH, Ahmad Nawaz, MBBS, Idan Goren, MD. P5459 - Advanced Therapies in IBD and Risk of Active Tuberculosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
