Tuesday Poster Session
Category: IBD
P5452 - High Rate of Initiation and Insurance Approval of Advanced Therapy in a Hub and Spoke Model Managing a Rural IBD Patient Population
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jamie M. Horrigan, MD
Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center
Lebanon, NH
Presenting Author(s)
Award: ACG Presidential Poster Award
Jamie M. Horrigan, MD1, Nicola Felicetti, MSN, RN2, Gina N. Manzi, PharmD2, Catherine Giguere-Rich, RD, LD, CNSC1, Jessica Salwen-Deremer, PhD3, Corey A. Siegel, MD, MS1
1Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 2Dartmouth Hitchcock Medical Center, Lebanon, NH; 3Section of Gastroenterology and Hepatology & Department of Psychiatry, Dartmouth-Hitchcock Medical Center, Lebanon, NH
Introduction: Recent data show that advanced therapy (biologics and small molecules) is underutilized in patients with inflammatory bowel disease (IBD). While this is an issue for all patients, it is magnified in rural regions where specialty teams are not widely available. RADIUS (Rural APPs Delivering IBD Care in the United States) is a multidisciplinary telehealth program focused on rural patients with IBD. It is organized as a hub and spoke model with rural community GI practices partnering with a comprehensive IBD program to co-manage patients. Our hypothesis was that the RADIUS model would lead to higher utilization of advanced therapy than is typically seen throughout the United States (US). Our aim was to measure the proportion of patients in RADIUS who received advanced therapy for IBD.
Methods: Community-based providers (primarily advanced practice providers) in rural practices in northern New England (Maine, New Hampshire, Vermont) refer patients with IBD to RADIUS. Patients have a one-time, two-hour virtual visit with the IBD specialist, psychologist, dietitian, pharmacist, and nurse coordinator. Data are collected at the initial visit and 6-12 months later to track if treatment recommendations were implemented and to assess change in patient confidence in their own disease management.
Results: 353 patients with IBD have been seen in the northern New England RADIUS hub. At the time of the visit, a change in therapy was recommended in 54.3% of patients. In those patients with medication changes recommended, 92.3% were approved by insurance, and the new medications were initiated in 84.8%. Average patient confidence in managing IBD increased from 5.8/10 pre-RADIUS visit to 7.4/10 at follow up (p< 0.001).
Discussion: Following RADIUS visits, a majority of patients were recommended to take advanced IBD therapy, and most had these medications approved by insurance and initiated on this treatment. This rate of advanced therapy use in RADIUS is significantly higher than has been reported in both rural and urban IBD populations in the US. We believe that increasing patient confidence in managing their IBD, having an IBD pharmacist part of every visit, and the partnership between spoke and hub centers has led to the high adoption of the treatment plan. Based on the early success of this model, three additional RADIUS hub and spoke regions have launched in Oregon, Colorado and Tennessee to serve their rural IBD patients.
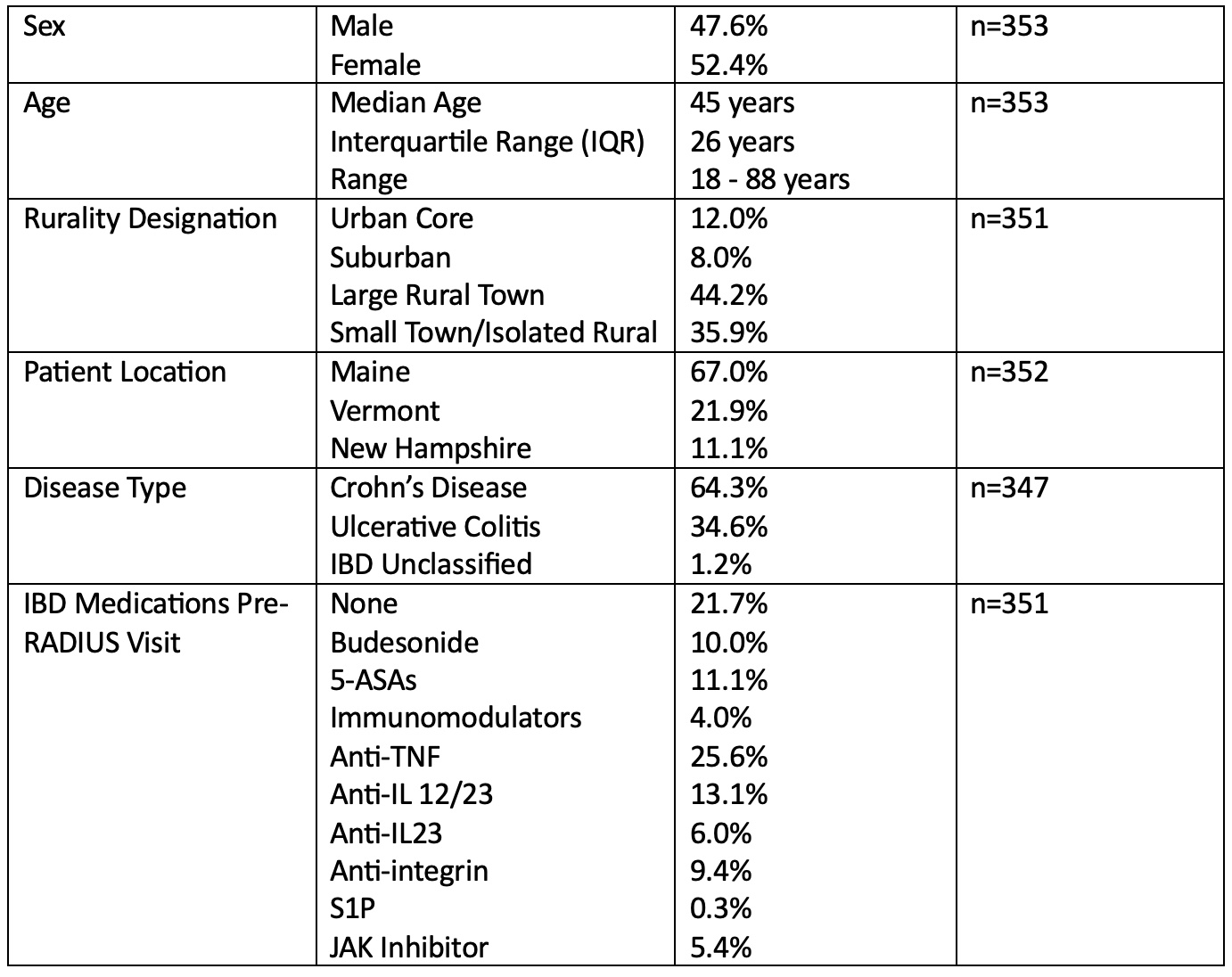
Figure: Table 1. RADIUS Patient Demographics
Disclosures:
Jamie Horrigan indicated no relevant financial relationships.
Nicola Felicetti indicated no relevant financial relationships.
Gina Manzi: Bristol Myers Squibb – Advisory Committee/Board Member. Janssen Pharmaceuticals – Advisory Committee/Board Member.
Catherine Giguere-Rich indicated no relevant financial relationships.
Jessica Salwen-Deremer: Buhlmann Diagnostics – Grant/Research Support. Eli Lilly – Consultant.
Corey A. Siegel: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boomerang – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Johnson and Johnson – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Napo Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Path Healthcare – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Prometheus Labs – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Trellus Health – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Jamie M. Horrigan, MD1, Nicola Felicetti, MSN, RN2, Gina N. Manzi, PharmD2, Catherine Giguere-Rich, RD, LD, CNSC1, Jessica Salwen-Deremer, PhD3, Corey A. Siegel, MD, MS1. P5452 - High Rate of Initiation and Insurance Approval of Advanced Therapy in a Hub and Spoke Model Managing a Rural IBD Patient Population, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Jamie M. Horrigan, MD1, Nicola Felicetti, MSN, RN2, Gina N. Manzi, PharmD2, Catherine Giguere-Rich, RD, LD, CNSC1, Jessica Salwen-Deremer, PhD3, Corey A. Siegel, MD, MS1
1Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 2Dartmouth Hitchcock Medical Center, Lebanon, NH; 3Section of Gastroenterology and Hepatology & Department of Psychiatry, Dartmouth-Hitchcock Medical Center, Lebanon, NH
Introduction: Recent data show that advanced therapy (biologics and small molecules) is underutilized in patients with inflammatory bowel disease (IBD). While this is an issue for all patients, it is magnified in rural regions where specialty teams are not widely available. RADIUS (Rural APPs Delivering IBD Care in the United States) is a multidisciplinary telehealth program focused on rural patients with IBD. It is organized as a hub and spoke model with rural community GI practices partnering with a comprehensive IBD program to co-manage patients. Our hypothesis was that the RADIUS model would lead to higher utilization of advanced therapy than is typically seen throughout the United States (US). Our aim was to measure the proportion of patients in RADIUS who received advanced therapy for IBD.
Methods: Community-based providers (primarily advanced practice providers) in rural practices in northern New England (Maine, New Hampshire, Vermont) refer patients with IBD to RADIUS. Patients have a one-time, two-hour virtual visit with the IBD specialist, psychologist, dietitian, pharmacist, and nurse coordinator. Data are collected at the initial visit and 6-12 months later to track if treatment recommendations were implemented and to assess change in patient confidence in their own disease management.
Results: 353 patients with IBD have been seen in the northern New England RADIUS hub. At the time of the visit, a change in therapy was recommended in 54.3% of patients. In those patients with medication changes recommended, 92.3% were approved by insurance, and the new medications were initiated in 84.8%. Average patient confidence in managing IBD increased from 5.8/10 pre-RADIUS visit to 7.4/10 at follow up (p< 0.001).
Discussion: Following RADIUS visits, a majority of patients were recommended to take advanced IBD therapy, and most had these medications approved by insurance and initiated on this treatment. This rate of advanced therapy use in RADIUS is significantly higher than has been reported in both rural and urban IBD populations in the US. We believe that increasing patient confidence in managing their IBD, having an IBD pharmacist part of every visit, and the partnership between spoke and hub centers has led to the high adoption of the treatment plan. Based on the early success of this model, three additional RADIUS hub and spoke regions have launched in Oregon, Colorado and Tennessee to serve their rural IBD patients.

Figure: Table 1. RADIUS Patient Demographics
Disclosures:
Jamie Horrigan indicated no relevant financial relationships.
Nicola Felicetti indicated no relevant financial relationships.
Gina Manzi: Bristol Myers Squibb – Advisory Committee/Board Member. Janssen Pharmaceuticals – Advisory Committee/Board Member.
Catherine Giguere-Rich indicated no relevant financial relationships.
Jessica Salwen-Deremer: Buhlmann Diagnostics – Grant/Research Support. Eli Lilly – Consultant.
Corey A. Siegel: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boomerang – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Johnson and Johnson – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Napo Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Path Healthcare – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Prometheus Labs – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Trellus Health – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Jamie M. Horrigan, MD1, Nicola Felicetti, MSN, RN2, Gina N. Manzi, PharmD2, Catherine Giguere-Rich, RD, LD, CNSC1, Jessica Salwen-Deremer, PhD3, Corey A. Siegel, MD, MS1. P5452 - High Rate of Initiation and Insurance Approval of Advanced Therapy in a Hub and Spoke Model Managing a Rural IBD Patient Population, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


