Tuesday Poster Session
Category: IBD
P5447 - Safety of COX-2 Inhibitor NSAIDs in Inflammatory Bowel Disease: A Propensity-Matched Cohort Study Using Real-World Data
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Tarek Odah, MD, MPH
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Tarek Odah, MD, MPH1, Hassan Ghoz, MD2, Jana G. Hashash, MD, MSc, FACG1, Francis A.. Farraye, MD, MSc, MACG1
1Mayo Clinic, Jacksonville, FL; 2University of Missouri Kansas City School of Medicine, Kansas City, MO
Introduction: Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for pain and inflammation but are often avoided in inflammatory bowel disease (IBD) due to concerns about worsening disease activity through COX-1 inhibition and disruption of mucosal integrity. Selective and preferential COX-2 inhibitors may mitigate this risk, but real-world safety data in ulcerative colitis and Crohn’s disease are limited.
Methods: We conducted a retrospective cohort study using the TriNetX platform to evaluate the association between prescribed NSAIDs and outcomes in ulcerative colitis (UC) and Crohn’s disease (CD). Patients were grouped as: (1) COX-2 inhibitors vs no NSAID, and (2) celecoxib vs meloxicam. Selective COX-2: celecoxib; preferential COX-2: meloxicam, nabumetone, etodolac. Propensity score matching was performed on demographics, comorbidities, recent ER/inpatient encounters, and markers of IBD severity, including recent use of steroids or IBD-specific therapies. The primary outcome was a composite of new intravenous steroids, oral steroids, new biologic/JAK/S1P initiation and/or fecal calprotectin ≥200 µg/g. Secondary outcomes included the individual components, IBD-related surgery, ICD-based documentation of UC or CD complications, ER/inpatient encounters, mortality, and—for CD—bowel obstruction or intervention for stricturing/perianal disease. Patients who experienced the study outcomes prior to the index prescription were excluded.
Results: Across four matched cohorts (two in UC and two in CD), sample sizes ranged from 805 to 3,009 per group. In UC, the primary composite outcome occurred in 5.2%–5.8% of patients across comparisons, with no significant differences observed. In CD, the primary outcome ranged from 5.9%–7.0%, also without significant associations. No statistically significant differences were observed in steroid or biologic/JAK/S1P use, fecal calprotectin, surgery, documented complications, hospitalization, or mortality.
Discussion: In this large real-world analysis, prescribed COX-2 inhibitors were not associated with increased risk of IBD activity. These findings suggest COX-2 inhibitors may be a reasonable option in appropriately selected patients with UC or CD.
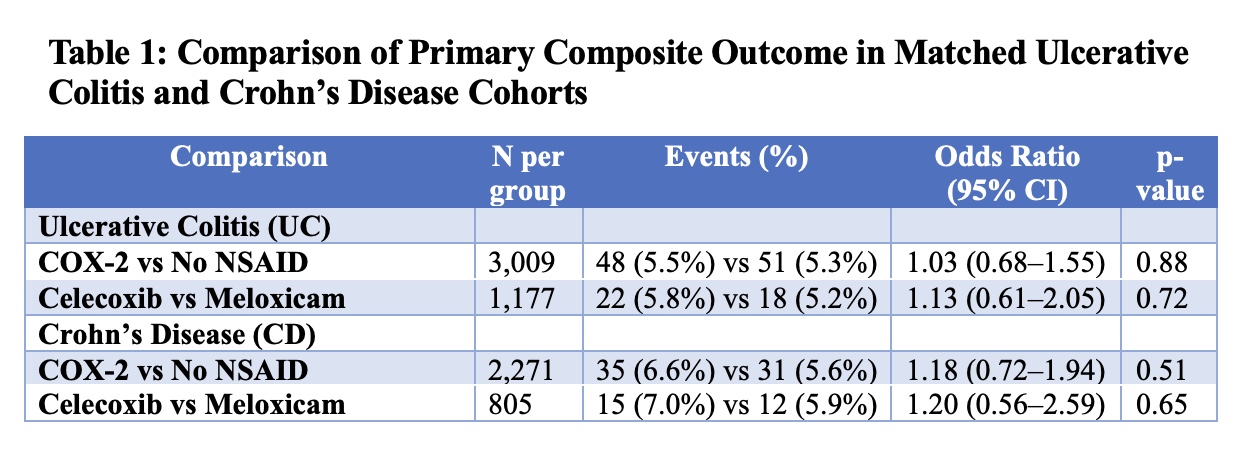
Figure: Table 1: Comparison of Primary Composite Outcome in Matched Ulcerative Colitis and Crohn’s Disease Cohorts by NSAID Exposure
Disclosures:
Tarek Odah indicated no relevant financial relationships.
Hassan Ghoz indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Tarek Odah, MD, MPH1, Hassan Ghoz, MD2, Jana G. Hashash, MD, MSc, FACG1, Francis A.. Farraye, MD, MSc, MACG1. P5447 - Safety of COX-2 Inhibitor NSAIDs in Inflammatory Bowel Disease: A Propensity-Matched Cohort Study Using Real-World Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Jacksonville, FL; 2University of Missouri Kansas City School of Medicine, Kansas City, MO
Introduction: Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for pain and inflammation but are often avoided in inflammatory bowel disease (IBD) due to concerns about worsening disease activity through COX-1 inhibition and disruption of mucosal integrity. Selective and preferential COX-2 inhibitors may mitigate this risk, but real-world safety data in ulcerative colitis and Crohn’s disease are limited.
Methods: We conducted a retrospective cohort study using the TriNetX platform to evaluate the association between prescribed NSAIDs and outcomes in ulcerative colitis (UC) and Crohn’s disease (CD). Patients were grouped as: (1) COX-2 inhibitors vs no NSAID, and (2) celecoxib vs meloxicam. Selective COX-2: celecoxib; preferential COX-2: meloxicam, nabumetone, etodolac. Propensity score matching was performed on demographics, comorbidities, recent ER/inpatient encounters, and markers of IBD severity, including recent use of steroids or IBD-specific therapies. The primary outcome was a composite of new intravenous steroids, oral steroids, new biologic/JAK/S1P initiation and/or fecal calprotectin ≥200 µg/g. Secondary outcomes included the individual components, IBD-related surgery, ICD-based documentation of UC or CD complications, ER/inpatient encounters, mortality, and—for CD—bowel obstruction or intervention for stricturing/perianal disease. Patients who experienced the study outcomes prior to the index prescription were excluded.
Results: Across four matched cohorts (two in UC and two in CD), sample sizes ranged from 805 to 3,009 per group. In UC, the primary composite outcome occurred in 5.2%–5.8% of patients across comparisons, with no significant differences observed. In CD, the primary outcome ranged from 5.9%–7.0%, also without significant associations. No statistically significant differences were observed in steroid or biologic/JAK/S1P use, fecal calprotectin, surgery, documented complications, hospitalization, or mortality.
Discussion: In this large real-world analysis, prescribed COX-2 inhibitors were not associated with increased risk of IBD activity. These findings suggest COX-2 inhibitors may be a reasonable option in appropriately selected patients with UC or CD.

Figure: Table 1: Comparison of Primary Composite Outcome in Matched Ulcerative Colitis and Crohn’s Disease Cohorts by NSAID Exposure
Disclosures:
Tarek Odah indicated no relevant financial relationships.
Hassan Ghoz indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Tarek Odah, MD, MPH1, Hassan Ghoz, MD2, Jana G. Hashash, MD, MSc, FACG1, Francis A.. Farraye, MD, MSc, MACG1. P5447 - Safety of COX-2 Inhibitor NSAIDs in Inflammatory Bowel Disease: A Propensity-Matched Cohort Study Using Real-World Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
