Tuesday Poster Session
Category: IBD
P5438 - Long-Term Comparative Outcomes of TNF-α Antagonists vs Vedolizumab as First-Line Therapy for Refractory Ulcerative Proctitis: A Propensity-Matched Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- AS
Ayushi Jayesh Shah, MD (she/her/hers)
SUNY Upstate Medical University
Syracuse, NY
Presenting Author(s)
Ayushi Jayesh. Shah, MD, Ahmad Nawaz, MBBS, Abdelkader Chaar, MD, Avleen Kaur, MD, Savio John, MD, FACG, Idan Goren, MD
SUNY Upstate Medical University, Syracuse, NY
Introduction: Ulcerative proctitis (UP), is a type of ulcerative colitis defined by inflammation limited to the rectum. A subset of patients with UP refractory to mesalamine may need biologic therapy. Patients with UP are frequently excluded from clinical trials of biologic therapies for UC, resulting in limited data to guide their use. This study evaluates the clinical outcomes of refractory UP patients treated with TNFi or VDZ as first-line biologic therapy.
Methods: We identifed adults with isolated UP on TriNetx using ICD-10 codes (Appendix). UP patients receiving TNFi or VDZ as first-line advanced therapy between 1/1995 to 1/2024 were included. Patients with left-sided colitis, pancolitis, Crohn’s disease, and autoimmune diseases were excluded. Complications of UP, defined as corticosteroid use, all-cause ER visits and hospitalizations, or colectomy, assessed at 6, 12, and 24 months after initiation of either TNFi or VDZ. Secondary outcomes included a sensitivity analysis of outcomes between adalimumab (ADA) and VDZ, and assessing the usage patterns of biologics in UP patients. Statistical analysis was done via TriNetX. Propensity score matching (PSM) was done for all known confounders (Figure 1). Adjusted odds ratios with 95% confidence intervals were calculated.
Results: Among 641 patients with UP exposed to advanced therapy, the most commonly used treatments were ADA (39%), infliximab (27%), and VDZ (26%). Ustekinumab was used in 12% of patients; tofacitinib and upadacitinib were each used in 6%. 328 patients were treated with TNFi and 135 with VDZ. Among TNFi, 192 received ADA and 136 received infliximab. Demographic and clinical characteristics were similar between groups (Figure 1). TNFi-treated patients had lower odds of ED visits and/or hospitalizations compared to VDZ at 6-months (14.3% vs 25%, p=0.03) and 12 months [16.1% vs. 35.2%, p= 0.01), before and after matching. No significant differences were observed between TNFi and VDZ in steroid use or colectomy (Figure 2). In 132 matched patients on ADA and VDZ, a similarly reduced risk of ED visits and/or hospitalizations at 6 and 12 months was seen in the ADA group.
Discussion: In conclusion, TNFi and VDZ demonstrate comparable risk of complications for refractory UP. TNFi may offer benefits in reducing ER visits and/or hospitalizations at 6 and 12-months, likely due to the faster onset of action. This finding was also seen for ADA vs VDZ. The rate of surgery in UP patients was low which may be expected due to limited disease.
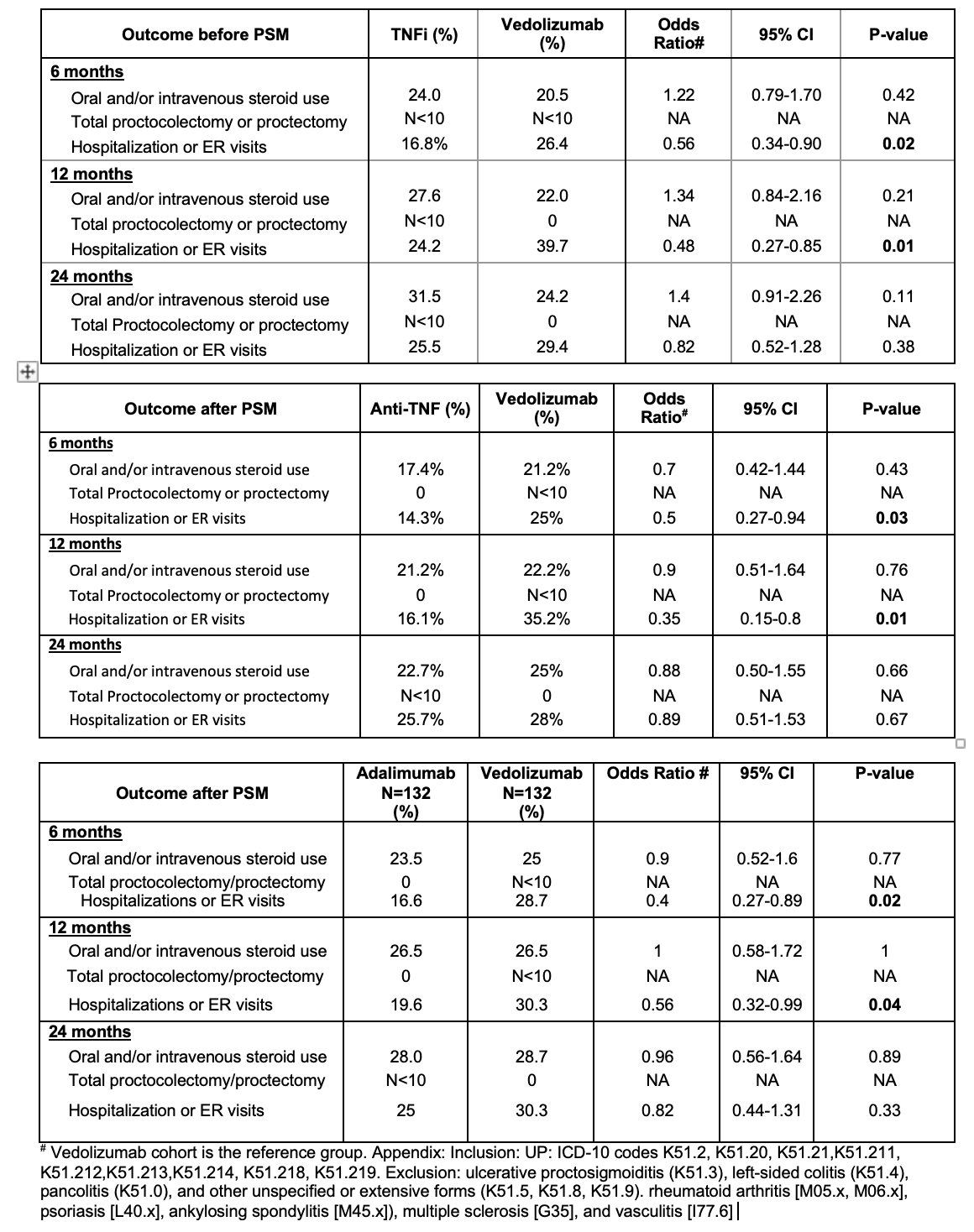
Figure: Baseline characteristics of patients in the TNFi and vedolizumab groups before and after propensity-score matching
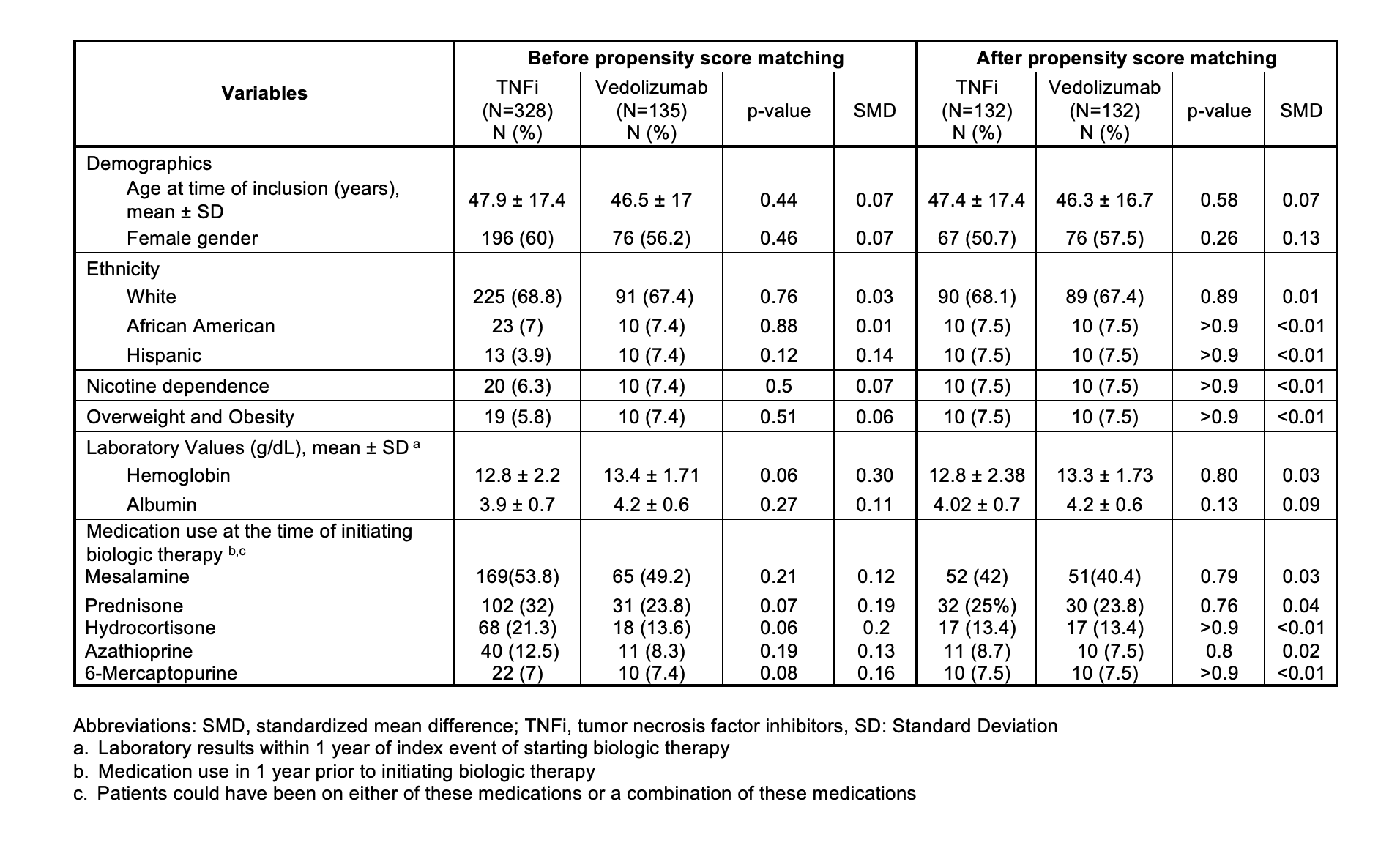
Figure: Table 2 and 3 show the comparison of outcomes between TNF-i and Vedolizumab groups at 6 months, 12 months and 24 months before and after propensity score matching, respectively.
Table 4 shows the sensitivity analysis of our findings between adalimumab and vedolizumab after PSM.
Appendix shows the ICD9/10 codes used
Disclosures:
Ayushi Shah indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Abdelkader Chaar indicated no relevant financial relationships.
Avleen Kaur indicated no relevant financial relationships.
Savio John indicated no relevant financial relationships.
Idan Goren indicated no relevant financial relationships.
Ayushi Jayesh. Shah, MD, Ahmad Nawaz, MBBS, Abdelkader Chaar, MD, Avleen Kaur, MD, Savio John, MD, FACG, Idan Goren, MD. P5438 - Long-Term Comparative Outcomes of TNF-α Antagonists vs Vedolizumab as First-Line Therapy for Refractory Ulcerative Proctitis: A Propensity-Matched Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
SUNY Upstate Medical University, Syracuse, NY
Introduction: Ulcerative proctitis (UP), is a type of ulcerative colitis defined by inflammation limited to the rectum. A subset of patients with UP refractory to mesalamine may need biologic therapy. Patients with UP are frequently excluded from clinical trials of biologic therapies for UC, resulting in limited data to guide their use. This study evaluates the clinical outcomes of refractory UP patients treated with TNFi or VDZ as first-line biologic therapy.
Methods: We identifed adults with isolated UP on TriNetx using ICD-10 codes (Appendix). UP patients receiving TNFi or VDZ as first-line advanced therapy between 1/1995 to 1/2024 were included. Patients with left-sided colitis, pancolitis, Crohn’s disease, and autoimmune diseases were excluded. Complications of UP, defined as corticosteroid use, all-cause ER visits and hospitalizations, or colectomy, assessed at 6, 12, and 24 months after initiation of either TNFi or VDZ. Secondary outcomes included a sensitivity analysis of outcomes between adalimumab (ADA) and VDZ, and assessing the usage patterns of biologics in UP patients. Statistical analysis was done via TriNetX. Propensity score matching (PSM) was done for all known confounders (Figure 1). Adjusted odds ratios with 95% confidence intervals were calculated.
Results: Among 641 patients with UP exposed to advanced therapy, the most commonly used treatments were ADA (39%), infliximab (27%), and VDZ (26%). Ustekinumab was used in 12% of patients; tofacitinib and upadacitinib were each used in 6%. 328 patients were treated with TNFi and 135 with VDZ. Among TNFi, 192 received ADA and 136 received infliximab. Demographic and clinical characteristics were similar between groups (Figure 1). TNFi-treated patients had lower odds of ED visits and/or hospitalizations compared to VDZ at 6-months (14.3% vs 25%, p=0.03) and 12 months [16.1% vs. 35.2%, p= 0.01), before and after matching. No significant differences were observed between TNFi and VDZ in steroid use or colectomy (Figure 2). In 132 matched patients on ADA and VDZ, a similarly reduced risk of ED visits and/or hospitalizations at 6 and 12 months was seen in the ADA group.
Discussion: In conclusion, TNFi and VDZ demonstrate comparable risk of complications for refractory UP. TNFi may offer benefits in reducing ER visits and/or hospitalizations at 6 and 12-months, likely due to the faster onset of action. This finding was also seen for ADA vs VDZ. The rate of surgery in UP patients was low which may be expected due to limited disease.

Figure: Baseline characteristics of patients in the TNFi and vedolizumab groups before and after propensity-score matching

Figure: Table 2 and 3 show the comparison of outcomes between TNF-i and Vedolizumab groups at 6 months, 12 months and 24 months before and after propensity score matching, respectively.
Table 4 shows the sensitivity analysis of our findings between adalimumab and vedolizumab after PSM.
Appendix shows the ICD9/10 codes used
Disclosures:
Ayushi Shah indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Abdelkader Chaar indicated no relevant financial relationships.
Avleen Kaur indicated no relevant financial relationships.
Savio John indicated no relevant financial relationships.
Idan Goren indicated no relevant financial relationships.
Ayushi Jayesh. Shah, MD, Ahmad Nawaz, MBBS, Abdelkader Chaar, MD, Avleen Kaur, MD, Savio John, MD, FACG, Idan Goren, MD. P5438 - Long-Term Comparative Outcomes of TNF-α Antagonists vs Vedolizumab as First-Line Therapy for Refractory Ulcerative Proctitis: A Propensity-Matched Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
