Tuesday Poster Session
Category: IBD
P5411 - Comparable Gastrointestinal Side Effects and Steroid Use in Anti-TNF–Naïve Ulcerative Colitis Patients Treated With Risankizumab or Guselkumab: A National Database Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- LN
Luis M. Nieto, MD (he/him/his)
Emory University School of Medicine
Atlanta, GA
Presenting Author(s)
Luis M.. Nieto, MD1, Sharon I.. Narvaez, MD2, Donghyun Ko, MD3, Do Han Kim, MD, MSc4, Olanrewaju Adeniran, MD5, Kenneth J.. Vega, MD, MHA6
1Emory University School of Medicine, Atlanta, GA; 2Piedmont Athens Regional, Atlanta, GA; 3Bridgeport Hospital, Bridgeport, CT; 4Icahn School of Medicine at Mount Sinai, New York, NY; 5West Virginia University Morgantown, Morgantown, WV; 6Prisma Health Midlands, Columbia, SC
Introduction: Interleukin-23 (IL-23) is a pro-inflammatory cytokine that plays a central role in the pathogenesis of Ulcerative Colitis (UC). Because of its key role in the inflammatory cascade, IL-23 is a major therapeutic target in UC. There is paucity of data comparing the use of Risankizumab versus Guselkumab, both IL-23 inhibitors. Therefore, we aim to evaluate the outcomes of anti-TNF naïve patients with UC taking Risankizumab or Guselkumab.
Methods: We performed a retrospective cohort study utilizing large population-based data from the TriNetX platform. We identified anti-TNF naïve patients with UC who received Risankizumab between January 1, 2024, and May 31, 2025. This cohort of patients was matched anti-TNF naïve patients with UC who received Guselkumab according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. The endpoints were gastrointestinal (GI) side effects (abdominal pain, nausea/vomiting, diarrhea, constipation and GERD) and intravenous (IV) or oral corticosteroid use. Logistic regression was used to estimate Odd Ratios (ORs).
Results: A total of 729 anti-TNF–naïve adult patients with UC were identified, 634 of these individuals were taking Risankizumab; 91 out of 634 (mean [SD] age, 43.6 [17.4] years; 38 [58.2%] female) were matched with 91 individuals (mean [SD] age, 44.9 [17.2] years; 49 [53.84%] female) who were taking Guselkumab. No differences were found in the incidence of GI side effects and use of IV or oral steroid use. The Risankizumab group notable for having 10 patients with nausea and vomiting when compared with no patients in the Guselkumab group; however, unable to measure of association between an exposure and an outcome due to lack of events in the Guselkumab group.
Discussion: In anti-TNF–naïve patients with UC, treatment with Risankizumab or Guselkumab resulted in similar rates of GI side effects except for nausea and vomiting seen only in patients using Risankizumab. There was no significant difference in the need for IV or oral corticosteroid use between the two therapies. These findings suggest comparable safety and steroid-sparing potential for both agents in this patient population. Further prospective studies are needed to determine the mechanism for these clinical findings.
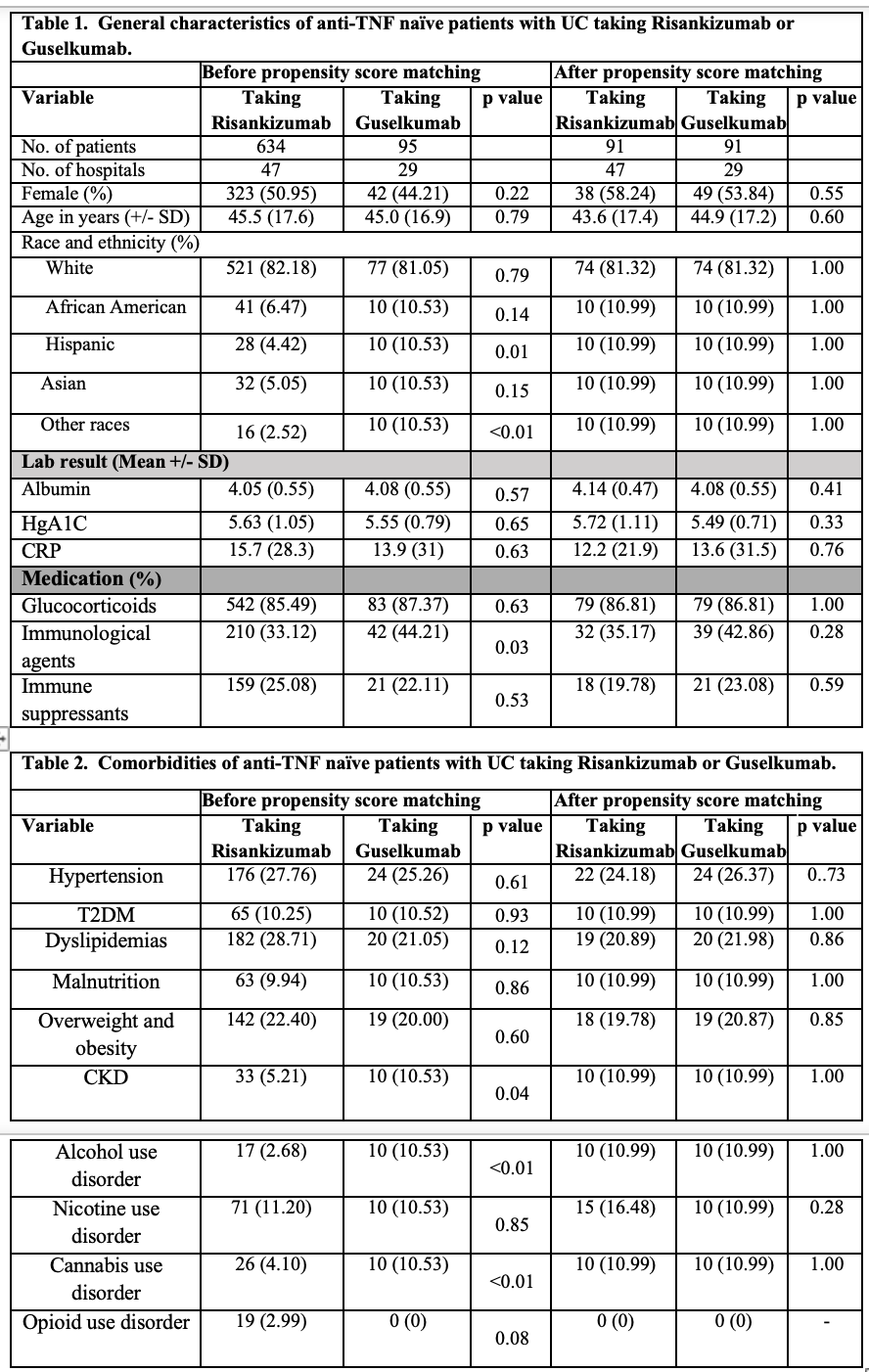
Figure: General characteristics and comorbidities of anti-TNF naïve patients with UC taking Risankizumab or Guselkumab.
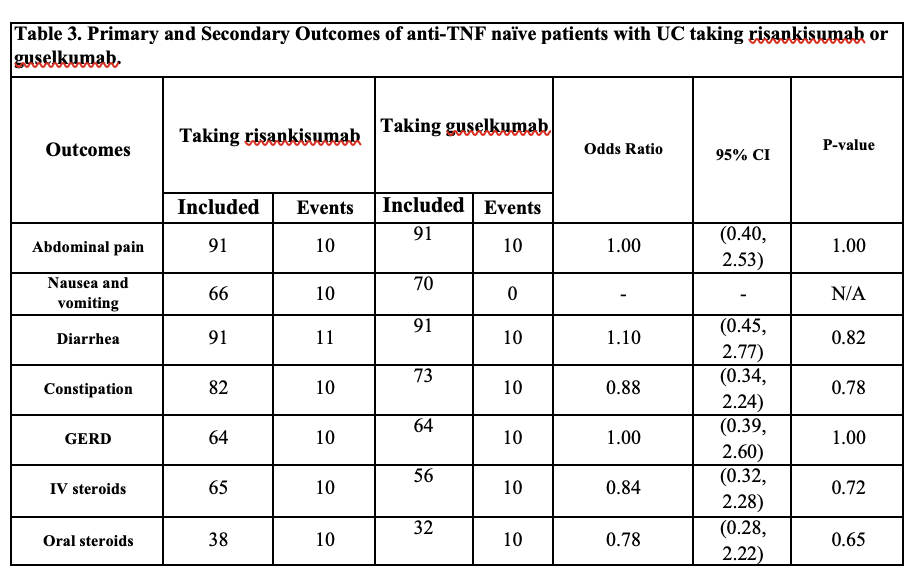
Figure: Outcomes of anti-TNF naïve patients with UC taking risankisumab or guselkumab.
Disclosures:
Luis Nieto indicated no relevant financial relationships.
Sharon Narvaez indicated no relevant financial relationships.
Donghyun Ko indicated no relevant financial relationships.
Do Han Kim indicated no relevant financial relationships.
Olanrewaju Adeniran indicated no relevant financial relationships.
Kenneth Vega: Phathom Pharmceuticals – Speakers Bureau.
Luis M.. Nieto, MD1, Sharon I.. Narvaez, MD2, Donghyun Ko, MD3, Do Han Kim, MD, MSc4, Olanrewaju Adeniran, MD5, Kenneth J.. Vega, MD, MHA6. P5411 - Comparable Gastrointestinal Side Effects and Steroid Use in Anti-TNF–Naïve Ulcerative Colitis Patients Treated With Risankizumab or Guselkumab: A National Database Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Emory University School of Medicine, Atlanta, GA; 2Piedmont Athens Regional, Atlanta, GA; 3Bridgeport Hospital, Bridgeport, CT; 4Icahn School of Medicine at Mount Sinai, New York, NY; 5West Virginia University Morgantown, Morgantown, WV; 6Prisma Health Midlands, Columbia, SC
Introduction: Interleukin-23 (IL-23) is a pro-inflammatory cytokine that plays a central role in the pathogenesis of Ulcerative Colitis (UC). Because of its key role in the inflammatory cascade, IL-23 is a major therapeutic target in UC. There is paucity of data comparing the use of Risankizumab versus Guselkumab, both IL-23 inhibitors. Therefore, we aim to evaluate the outcomes of anti-TNF naïve patients with UC taking Risankizumab or Guselkumab.
Methods: We performed a retrospective cohort study utilizing large population-based data from the TriNetX platform. We identified anti-TNF naïve patients with UC who received Risankizumab between January 1, 2024, and May 31, 2025. This cohort of patients was matched anti-TNF naïve patients with UC who received Guselkumab according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. The endpoints were gastrointestinal (GI) side effects (abdominal pain, nausea/vomiting, diarrhea, constipation and GERD) and intravenous (IV) or oral corticosteroid use. Logistic regression was used to estimate Odd Ratios (ORs).
Results: A total of 729 anti-TNF–naïve adult patients with UC were identified, 634 of these individuals were taking Risankizumab; 91 out of 634 (mean [SD] age, 43.6 [17.4] years; 38 [58.2%] female) were matched with 91 individuals (mean [SD] age, 44.9 [17.2] years; 49 [53.84%] female) who were taking Guselkumab. No differences were found in the incidence of GI side effects and use of IV or oral steroid use. The Risankizumab group notable for having 10 patients with nausea and vomiting when compared with no patients in the Guselkumab group; however, unable to measure of association between an exposure and an outcome due to lack of events in the Guselkumab group.
Discussion: In anti-TNF–naïve patients with UC, treatment with Risankizumab or Guselkumab resulted in similar rates of GI side effects except for nausea and vomiting seen only in patients using Risankizumab. There was no significant difference in the need for IV or oral corticosteroid use between the two therapies. These findings suggest comparable safety and steroid-sparing potential for both agents in this patient population. Further prospective studies are needed to determine the mechanism for these clinical findings.

Figure: General characteristics and comorbidities of anti-TNF naïve patients with UC taking Risankizumab or Guselkumab.

Figure: Outcomes of anti-TNF naïve patients with UC taking risankisumab or guselkumab.
Disclosures:
Luis Nieto indicated no relevant financial relationships.
Sharon Narvaez indicated no relevant financial relationships.
Donghyun Ko indicated no relevant financial relationships.
Do Han Kim indicated no relevant financial relationships.
Olanrewaju Adeniran indicated no relevant financial relationships.
Kenneth Vega: Phathom Pharmceuticals – Speakers Bureau.
Luis M.. Nieto, MD1, Sharon I.. Narvaez, MD2, Donghyun Ko, MD3, Do Han Kim, MD, MSc4, Olanrewaju Adeniran, MD5, Kenneth J.. Vega, MD, MHA6. P5411 - Comparable Gastrointestinal Side Effects and Steroid Use in Anti-TNF–Naïve Ulcerative Colitis Patients Treated With Risankizumab or Guselkumab: A National Database Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
