Tuesday Poster Session
Category: IBD
P5404 - Long-Term Outcome of Patients With Inflammatory Bowel Disease and Immunogenicity to Adalimumab
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Alessandra Saraga, MD
Beth Israel Deaconess Medical Center, Harvard Medical School
Boston, MA
Presenting Author(s)
Alessandra Saraga, MD1, Ajay Gade, MD2, Tina Deyhim, MD2, Grace Geeganage, BSc2, Samantha Zullow, MD2, Loren Rabinowitz, MD2, Laurie Grossberg, MD2, Adam Cheifetz, MD, FACG2, Konstantinos Papamichail, MD, PhD2
1Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA; 2Beth Israel Deaconess Medical Center, Boston, MA
Introduction: There are limited data regarding the association of antibodies to adalimumab (ATA) and long-term outcomes in patients with inflammatory bowel disease (IBD). The primary aim of this study was to investigate long-term outcomes of patients with IBD and immunogenicity to adalimumab and identify variables associated with treatment failure. A secondary outcome was to compare patients with ATA and either detectable or undetectable adalimumab concentrations in terms of treatment failure.
Methods: This single-center, retrospective, cohort study included consecutive patients with IBD treated with ADM who had positive ATA while undergoing TDM with a drug tolerant assay (homogenous mobility shift assay) between November, 2013, and February, 2023. A time-to-event analysis was performed for treatment failure, defined as drug discontinuation due to primary non-response (PNR), loss of response (LOR) or a serious injection site reaction (SIR). Patients were followed from first TDM with positive ATA until drug discontinuation or the end of the follow up period (May 2024). A receiver operating characteristic (ROC) curve analysis was performed to identify an ADM concentration threshold associated with treatment failure.
Results: The study population consisted of 58 patients with IBD (n=39, 67% with Crohn’s disease). Patients were followed for a median of 39.3 months [interquartile range (IQR) 39.3 (24.7-53)]. Thirty-six patients (62%) had treatment failure (PNR, n=6; LOR, n=28; SIR, n=2, Figure 1). Multiple COX regression analysis identified elevated C-reactive protein (CRP) at first TDM with positive ATA [hazard ratio (HR): 1.05, 95% confidence interval (CI): 1.01-1.09, p=0.008] and higher ATA titers (HR: 1.05, 95%CI 1.03-1.07, p< 0.001) as variables associated with treatment failure. ROC analysis identified an ATA titer threshold of 5.2 U/mL as distinguishing patients with or without treatment failure (area under the ROC curve: 0.705; 95%CI: 0.569-0.841; p=0.009; sensitivity: 64%; specificity: 82%). Patients with ATA and undetectable drug concentrations had higher treatment failure than patients with ATA and detectable drug concentration (Log Rank p=0.009, Figure 2).
Discussion: In this cohort study, most patients with positive ATA experienced treatment failure, particularly those with undetectable compared to patients with detectable drug concentrations. Elevated CRP and higher ATA titer were associated with treatment failure
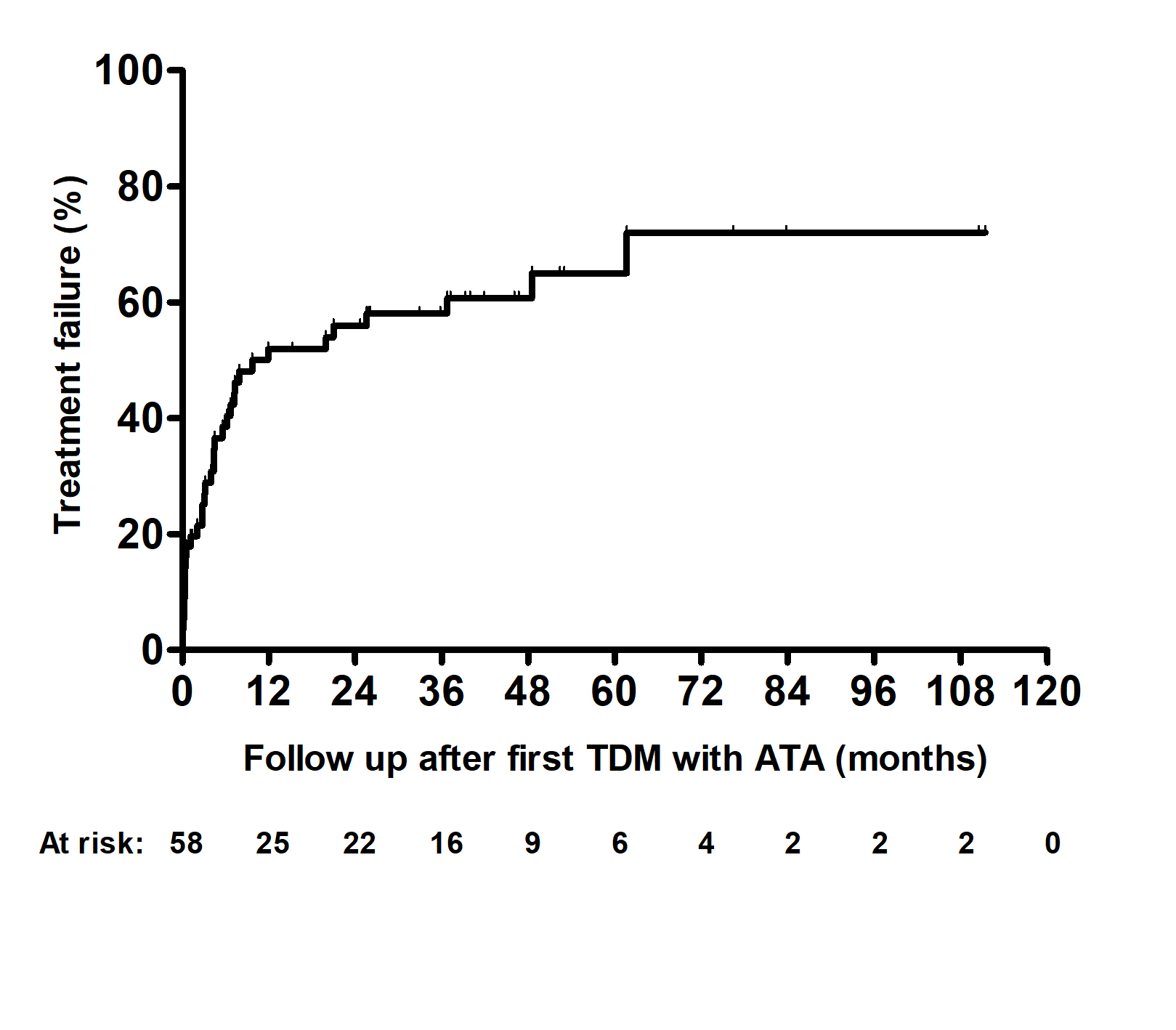
Figure: Kaplan–Meier cumulative probability curve of treatment failure in patients with IBD and anti-drug antibodies treated with adalimumab
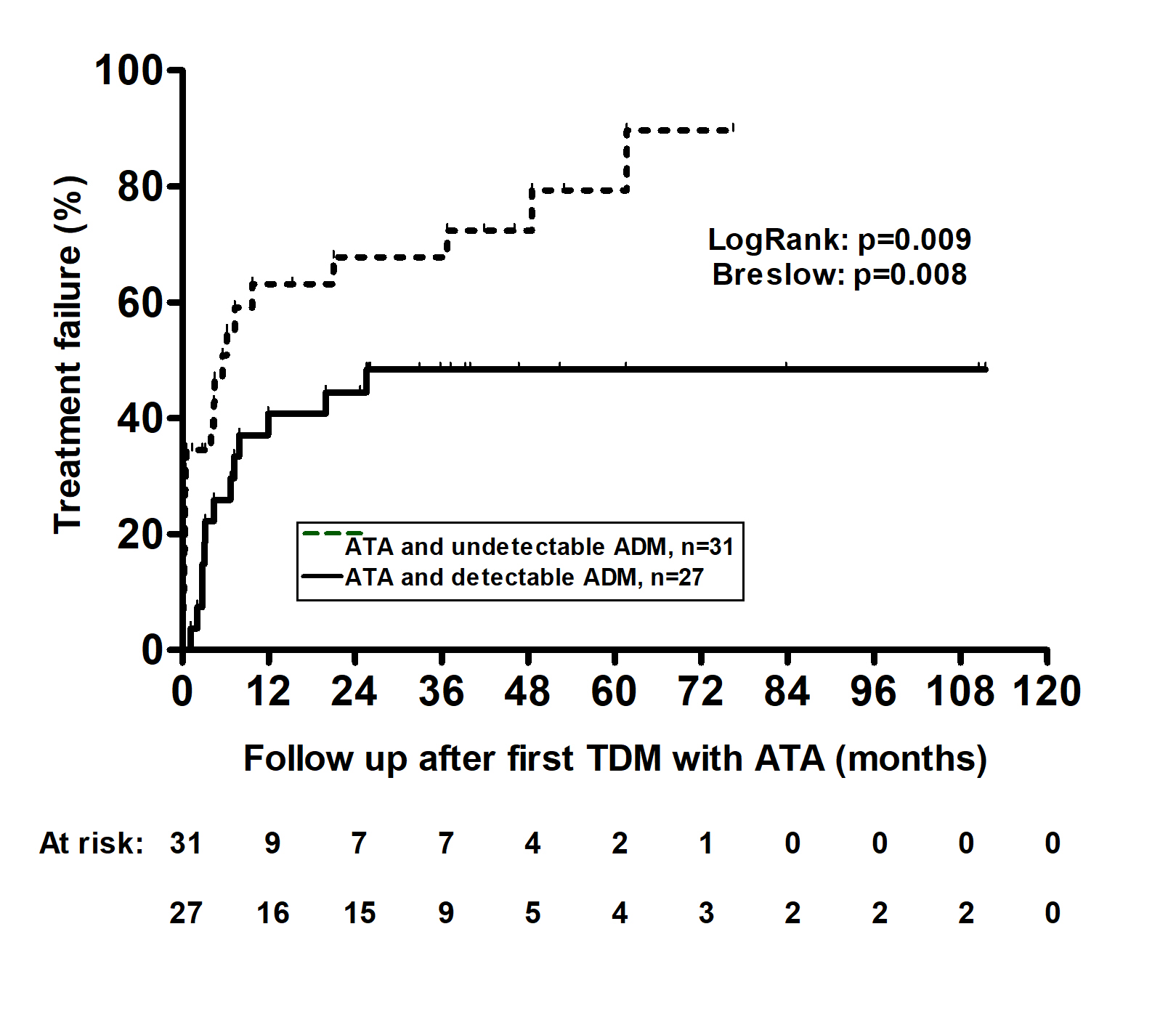
Figure: Kaplan–Meier cumulative probability curves of treatment failure in patients with IBD and antibodies to adalimumab with either undetectable (dotted line) or detectable (solid line) drug concentrations
Disclosures:
Alessandra Saraga indicated no relevant financial relationships.
Ajay Gade indicated no relevant financial relationships.
Tina Deyhim indicated no relevant financial relationships.
Grace Geeganage indicated no relevant financial relationships.
Samantha Zullow: Johnson & Johnson – Advisory Committee/Board Member.
Loren Rabinowitz indicated no relevant financial relationships.
Laurie Grossberg: Pfizer – Advisory Committee/Board Member.
Adam Cheifetz: Abbvie – Consultant, Speakers Bureau. Adiso – Consultant. Aegirbio – Advisory Committee/Board Member. Artizan – Advisory Committee/Board Member. BMS – Consultant, Speakers Bureau. Celltrion Inc – Advisory Committee/Board Member, Consultant. Clario – Consultant. Eli Lilly – Consultant. Food is Good – Consultant. Fresenius Kabi – Consultant. Fzata – Consultant. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. ProciseDx – Advisory Committee/Board Member. Prometheus – Advisory Committee/Board Member. Samsung – Consultant. Spherix – Consultant.
Konstantinos Papamichail: Celltrion Inc – Advisory Committee/Board Member. Prometheus Laboratories Inc – Consultant, Speakers Bureau.
Alessandra Saraga, MD1, Ajay Gade, MD2, Tina Deyhim, MD2, Grace Geeganage, BSc2, Samantha Zullow, MD2, Loren Rabinowitz, MD2, Laurie Grossberg, MD2, Adam Cheifetz, MD, FACG2, Konstantinos Papamichail, MD, PhD2. P5404 - Long-Term Outcome of Patients With Inflammatory Bowel Disease and Immunogenicity to Adalimumab, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA; 2Beth Israel Deaconess Medical Center, Boston, MA
Introduction: There are limited data regarding the association of antibodies to adalimumab (ATA) and long-term outcomes in patients with inflammatory bowel disease (IBD). The primary aim of this study was to investigate long-term outcomes of patients with IBD and immunogenicity to adalimumab and identify variables associated with treatment failure. A secondary outcome was to compare patients with ATA and either detectable or undetectable adalimumab concentrations in terms of treatment failure.
Methods: This single-center, retrospective, cohort study included consecutive patients with IBD treated with ADM who had positive ATA while undergoing TDM with a drug tolerant assay (homogenous mobility shift assay) between November, 2013, and February, 2023. A time-to-event analysis was performed for treatment failure, defined as drug discontinuation due to primary non-response (PNR), loss of response (LOR) or a serious injection site reaction (SIR). Patients were followed from first TDM with positive ATA until drug discontinuation or the end of the follow up period (May 2024). A receiver operating characteristic (ROC) curve analysis was performed to identify an ADM concentration threshold associated with treatment failure.
Results: The study population consisted of 58 patients with IBD (n=39, 67% with Crohn’s disease). Patients were followed for a median of 39.3 months [interquartile range (IQR) 39.3 (24.7-53)]. Thirty-six patients (62%) had treatment failure (PNR, n=6; LOR, n=28; SIR, n=2, Figure 1). Multiple COX regression analysis identified elevated C-reactive protein (CRP) at first TDM with positive ATA [hazard ratio (HR): 1.05, 95% confidence interval (CI): 1.01-1.09, p=0.008] and higher ATA titers (HR: 1.05, 95%CI 1.03-1.07, p< 0.001) as variables associated with treatment failure. ROC analysis identified an ATA titer threshold of 5.2 U/mL as distinguishing patients with or without treatment failure (area under the ROC curve: 0.705; 95%CI: 0.569-0.841; p=0.009; sensitivity: 64%; specificity: 82%). Patients with ATA and undetectable drug concentrations had higher treatment failure than patients with ATA and detectable drug concentration (Log Rank p=0.009, Figure 2).
Discussion: In this cohort study, most patients with positive ATA experienced treatment failure, particularly those with undetectable compared to patients with detectable drug concentrations. Elevated CRP and higher ATA titer were associated with treatment failure

Figure: Kaplan–Meier cumulative probability curve of treatment failure in patients with IBD and anti-drug antibodies treated with adalimumab

Figure: Kaplan–Meier cumulative probability curves of treatment failure in patients with IBD and antibodies to adalimumab with either undetectable (dotted line) or detectable (solid line) drug concentrations
Disclosures:
Alessandra Saraga indicated no relevant financial relationships.
Ajay Gade indicated no relevant financial relationships.
Tina Deyhim indicated no relevant financial relationships.
Grace Geeganage indicated no relevant financial relationships.
Samantha Zullow: Johnson & Johnson – Advisory Committee/Board Member.
Loren Rabinowitz indicated no relevant financial relationships.
Laurie Grossberg: Pfizer – Advisory Committee/Board Member.
Adam Cheifetz: Abbvie – Consultant, Speakers Bureau. Adiso – Consultant. Aegirbio – Advisory Committee/Board Member. Artizan – Advisory Committee/Board Member. BMS – Consultant, Speakers Bureau. Celltrion Inc – Advisory Committee/Board Member, Consultant. Clario – Consultant. Eli Lilly – Consultant. Food is Good – Consultant. Fresenius Kabi – Consultant. Fzata – Consultant. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. ProciseDx – Advisory Committee/Board Member. Prometheus – Advisory Committee/Board Member. Samsung – Consultant. Spherix – Consultant.
Konstantinos Papamichail: Celltrion Inc – Advisory Committee/Board Member. Prometheus Laboratories Inc – Consultant, Speakers Bureau.
Alessandra Saraga, MD1, Ajay Gade, MD2, Tina Deyhim, MD2, Grace Geeganage, BSc2, Samantha Zullow, MD2, Loren Rabinowitz, MD2, Laurie Grossberg, MD2, Adam Cheifetz, MD, FACG2, Konstantinos Papamichail, MD, PhD2. P5404 - Long-Term Outcome of Patients With Inflammatory Bowel Disease and Immunogenicity to Adalimumab, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
