Tuesday Poster Session
Category: IBD
P5393 - Adherence to STRIDE-II Treat-to-Target Guidelines in Recent FDA-Approved IBD Drug Trials: A Comparative Review
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Harshavardhan Sanekommu, MD (he/him/his)
Hackensack Meridian Jersey Shore University Medical Center
Neptune City, NJ
Presenting Author(s)
Harshavardhan Sanekommu, MD1, Idan Grossmann, MD1, Karolina Kaczmarczyk, MD, MPH1, Vera Hapshy, DO2, Lee Peng, MD, PhD3
1Hackensack Meridian Jersey Shore University Medical Center, Neptune City, NJ; 2Hackensack Meridian JSUMC, Neptune City, NJ; 3Hackensack Meridian Health, Neptune, NJ
Introduction: The STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) consensus guidelines, published in 2021 by the International Organization for the Study of IBD (IOIBD), established a comprehensive treat-to-target strategy emphasizing clinical remission, endoscopic healing, normalization of biomarkers (CRP, fecal calprotectin), and quality of life (QoL) as key therapeutic endpoints. Since the release of STRIDE-II, multiple new therapies for Crohn’s disease (CD) and ulcerative colitis (UC) have received FDA approval. It remains unclear to what extent pivotal trials leading to these approvals adhered to STRIDE-II targets.
Methods: A structured review was conducted of Phase 3 clinical trials that supported FDA approval of the following agents between 2021 and 2025: mirikizumab (Omvoh), guselkumab (Tremfya), upadacitinib (Rinvoq), etrasimod (Velsipity), infliximab-dyyb (Zymfentra), and subcutaneous vedolizumab (Entyvio SC). Trials were assessed for inclusion of STRIDE-II endpoints: (1) clinical remission, (2) endoscopic healing, (3) biomarker normalization, and (4) validated QoL assessment (e.g., IBDQ, PROMIS).
Results: All six trials included clinical remission and endoscopic healing as primary or co-primary endpoints, consistent with STRIDE-II recommendations. However, none of the pivotal trials included biomarker normalization or validated QoL instruments as formal endpoints. Mirikizumab’s LUCENT trials uniquely evaluated bowel urgency as a patient-reported symptom, a symptomatic target identified in STRIDE-II. Trials of subcutaneous formulations (Zymfentra and Entyvio SC) emphasized maintenance efficacy and convenience but lacked comprehensive disease activity or patient-centered outcomes.
Discussion: Recent FDA-approved IBD therapies have shown strong alignment with core STRIDE-II targets of clinical and endoscopic remission. However, the consistent omission of biomarker normalization and QoL assessment represents a significant gap in adherence to STRIDE-II’s full treat-to-target framework. Future trials should integrate biochemical and patient-reported outcomes to fully align with STRIDE-II and enhance patient-centered care in IBD.
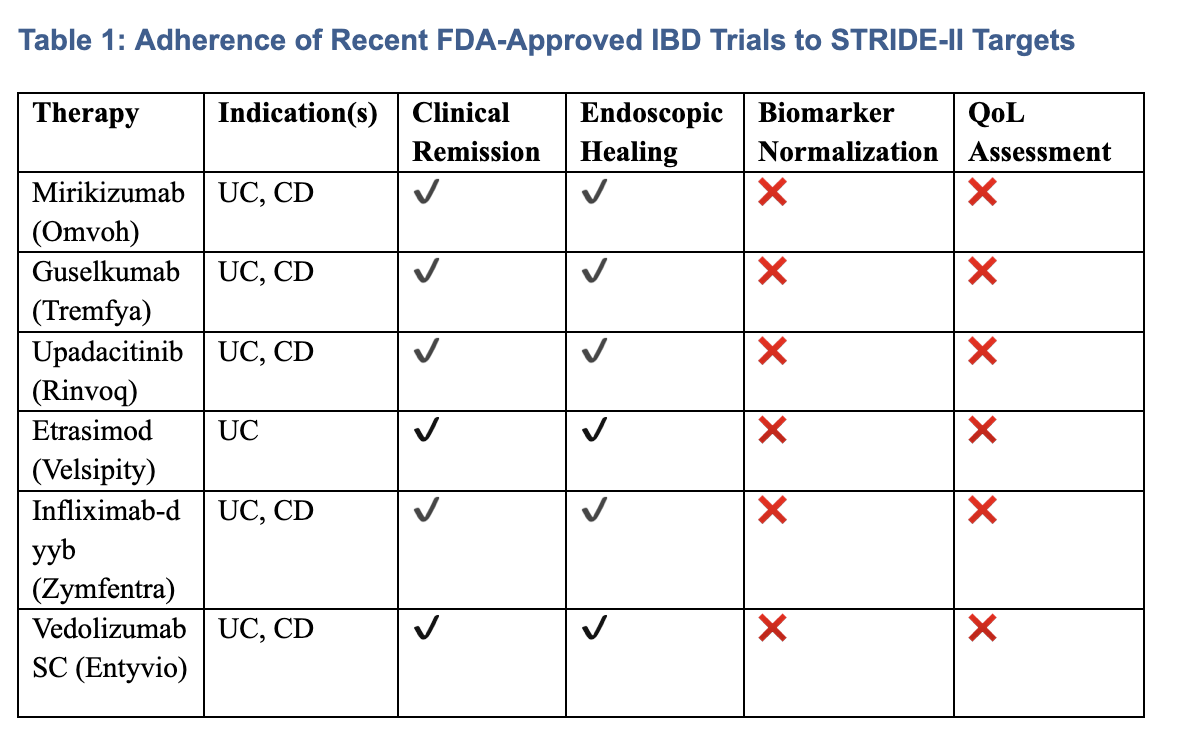
Figure: Table 1: Adherence of Recent FDA-Approved IBD Trials to STRIDE-II Targets
Disclosures:
Harshavardhan Sanekommu indicated no relevant financial relationships.
Idan Grossmann indicated no relevant financial relationships.
Karolina Kaczmarczyk indicated no relevant financial relationships.
Vera Hapshy indicated no relevant financial relationships.
Lee Peng indicated no relevant financial relationships.
Harshavardhan Sanekommu, MD1, Idan Grossmann, MD1, Karolina Kaczmarczyk, MD, MPH1, Vera Hapshy, DO2, Lee Peng, MD, PhD3. P5393 - Adherence to STRIDE-II Treat-to-Target Guidelines in Recent FDA-Approved IBD Drug Trials: A Comparative Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Hackensack Meridian Jersey Shore University Medical Center, Neptune City, NJ; 2Hackensack Meridian JSUMC, Neptune City, NJ; 3Hackensack Meridian Health, Neptune, NJ
Introduction: The STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) consensus guidelines, published in 2021 by the International Organization for the Study of IBD (IOIBD), established a comprehensive treat-to-target strategy emphasizing clinical remission, endoscopic healing, normalization of biomarkers (CRP, fecal calprotectin), and quality of life (QoL) as key therapeutic endpoints. Since the release of STRIDE-II, multiple new therapies for Crohn’s disease (CD) and ulcerative colitis (UC) have received FDA approval. It remains unclear to what extent pivotal trials leading to these approvals adhered to STRIDE-II targets.
Methods: A structured review was conducted of Phase 3 clinical trials that supported FDA approval of the following agents between 2021 and 2025: mirikizumab (Omvoh), guselkumab (Tremfya), upadacitinib (Rinvoq), etrasimod (Velsipity), infliximab-dyyb (Zymfentra), and subcutaneous vedolizumab (Entyvio SC). Trials were assessed for inclusion of STRIDE-II endpoints: (1) clinical remission, (2) endoscopic healing, (3) biomarker normalization, and (4) validated QoL assessment (e.g., IBDQ, PROMIS).
Results: All six trials included clinical remission and endoscopic healing as primary or co-primary endpoints, consistent with STRIDE-II recommendations. However, none of the pivotal trials included biomarker normalization or validated QoL instruments as formal endpoints. Mirikizumab’s LUCENT trials uniquely evaluated bowel urgency as a patient-reported symptom, a symptomatic target identified in STRIDE-II. Trials of subcutaneous formulations (Zymfentra and Entyvio SC) emphasized maintenance efficacy and convenience but lacked comprehensive disease activity or patient-centered outcomes.
Discussion: Recent FDA-approved IBD therapies have shown strong alignment with core STRIDE-II targets of clinical and endoscopic remission. However, the consistent omission of biomarker normalization and QoL assessment represents a significant gap in adherence to STRIDE-II’s full treat-to-target framework. Future trials should integrate biochemical and patient-reported outcomes to fully align with STRIDE-II and enhance patient-centered care in IBD.

Figure: Table 1: Adherence of Recent FDA-Approved IBD Trials to STRIDE-II Targets
Disclosures:
Harshavardhan Sanekommu indicated no relevant financial relationships.
Idan Grossmann indicated no relevant financial relationships.
Karolina Kaczmarczyk indicated no relevant financial relationships.
Vera Hapshy indicated no relevant financial relationships.
Lee Peng indicated no relevant financial relationships.
Harshavardhan Sanekommu, MD1, Idan Grossmann, MD1, Karolina Kaczmarczyk, MD, MPH1, Vera Hapshy, DO2, Lee Peng, MD, PhD3. P5393 - Adherence to STRIDE-II Treat-to-Target Guidelines in Recent FDA-Approved IBD Drug Trials: A Comparative Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
