Tuesday Poster Session
Category: IBD
P5390 - Pharmacokinetics and Relative Bioavailability of an Investigational Hydrocortisone Acetate Suppository Administered With a Novel FDA-Cleared Applicator Versus Hydrocortisone Liquid Enema
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- ME
Mark Ensign
Cristcot
Concord, MA
Presenting Author(s)
Mark Ensign, 1, Raj Devarajan, MD2, Jennifer Davagian, 1
1Cristcot, Concord, MA; 2Shivkar LLC, Acton, MA
Introduction: Rectally-administered hydrocortisone (HC) therapy is used to treat inflammatory conditions of the distal colon. This phase 1 study was designed to characterize the pharmacokinetic (PK) profile of a novel, investigational 90 mg hydrocortisone acetate (HCA) suppository formulation administered with a proprietary FDA-cleared applicator vs 100 mg HC liquid rectal enema.
Methods: In this single-center, open-label, single-dose, 2-way crossover study conducted in healthy adults, study participants were randomized to 1 of 2 sequences with a 7-day washout between treatments. Under the Investigational New Drug exemption, the study treatments, HC 100 mg/60 mL liquid enema and 90 mg HCA suppository using the applicator, were administered to study participants by site personnel after an overnight fast. HCA hydrolyzes in vivo to HC (exogenous cortisol). Study participants received 4 mg oral dexamethasone (DEX) 10 hours before each treatment to suppress endogenous cortisol. Plasma PK parameters were assessed using non-compartmental methods. Assay limit of quantification was 0.4 ng/mL for HCA and 4 ng/mL for HC.
Results: Sixteen study participants (8 male/8 female) were enrolled and completed the study (mean age, 30 y; mean weight, 73 kg). HCA was not detected in any sample. DEX suppression resulted in pre-dose HC plasma concentrations (Cp) < 11 ng/mL in all participants; however, in 13 sessions, HC Cp increased at the 24-hour sample (and in 2 sessions at 16 hours), presumably due to dissipation of DEX suppression. As a result, AUC0-∞ could be assessed in only 9 participants. The mean maximum Cp (Cmax) was 194.0 ng/mL for HCA and 392.5 ng/mL for HC (Table). The mean area under the Cp vs time curve from pre-dose to the time of the last quantifiable concentration (AUC0-t) was 3125.0 h·ng/mL for HCA and 3611.0 h·ng/mL for HC. Mean HCA:HC ratios for Cmax and AUC0-t were 0.50 and 0.86, respectively. Cp peaks occurred at 8.0 h and 2.5 h (median) for HCA and HC, respectively (P< 0.0001; Figure). Half-life was 11.7 h for HCA and 5.7 h for HC. Four adverse events (AEs), all mild, were reported; no AEs were serious.
Discussion: The novel, investigational 90 mg HCA suppository administered using the proprietary FDA-cleared applicator demonstrated rapid drug release, sustained HC Cp, and was well tolerated. The unique release and absorption profile of the investigational 90 mg HCA suppository warrants further clinical development for management of inflammatory bowel disease.
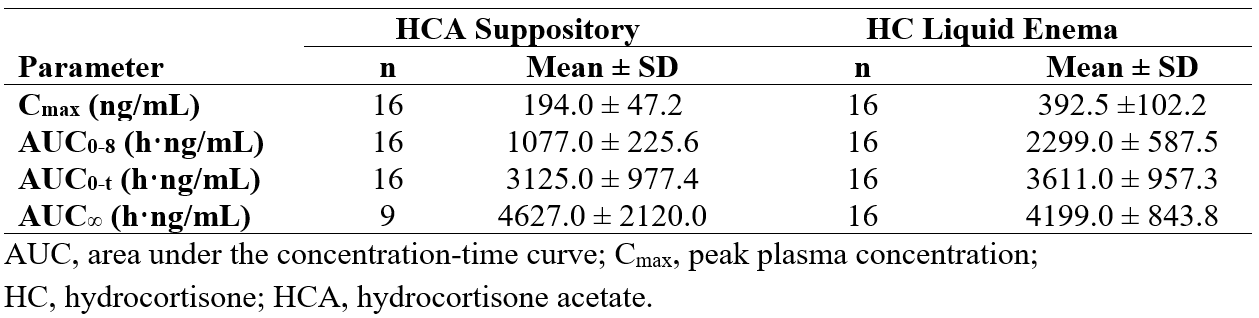
Figure: Table. HC Pharmacokinetic Parameters in Plasma
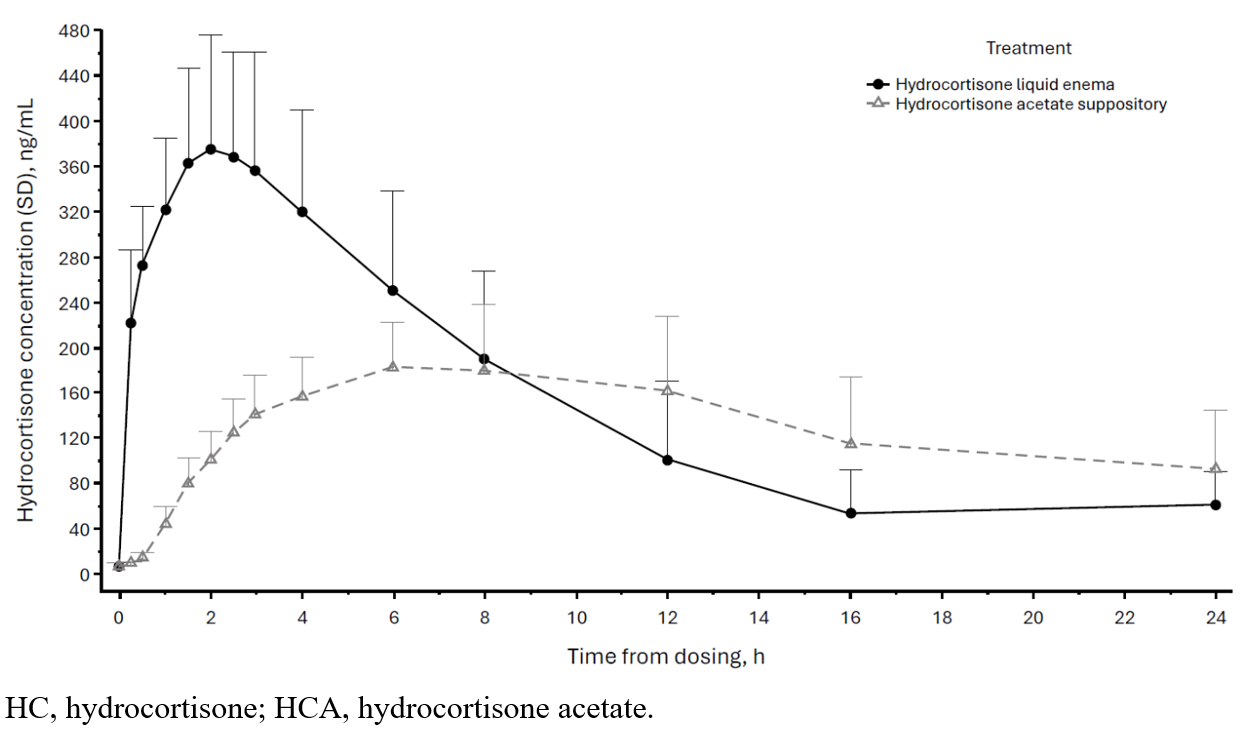
Figure: Figure. Baseline-Corrected Plasma HC Concentration Over Time for HCA Suppository Versus HC Liquid Enema
Disclosures:
Mark Ensign: Cristcot – Employee.
Raj Devarajan: Cristcot – Consultant.
Jennifer Davagian: Cristcot – Employee, Equity.
Mark Ensign, 1, Raj Devarajan, MD2, Jennifer Davagian, 1. P5390 - Pharmacokinetics and Relative Bioavailability of an Investigational Hydrocortisone Acetate Suppository Administered With a Novel FDA-Cleared Applicator Versus Hydrocortisone Liquid Enema, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cristcot, Concord, MA; 2Shivkar LLC, Acton, MA
Introduction: Rectally-administered hydrocortisone (HC) therapy is used to treat inflammatory conditions of the distal colon. This phase 1 study was designed to characterize the pharmacokinetic (PK) profile of a novel, investigational 90 mg hydrocortisone acetate (HCA) suppository formulation administered with a proprietary FDA-cleared applicator vs 100 mg HC liquid rectal enema.
Methods: In this single-center, open-label, single-dose, 2-way crossover study conducted in healthy adults, study participants were randomized to 1 of 2 sequences with a 7-day washout between treatments. Under the Investigational New Drug exemption, the study treatments, HC 100 mg/60 mL liquid enema and 90 mg HCA suppository using the applicator, were administered to study participants by site personnel after an overnight fast. HCA hydrolyzes in vivo to HC (exogenous cortisol). Study participants received 4 mg oral dexamethasone (DEX) 10 hours before each treatment to suppress endogenous cortisol. Plasma PK parameters were assessed using non-compartmental methods. Assay limit of quantification was 0.4 ng/mL for HCA and 4 ng/mL for HC.
Results: Sixteen study participants (8 male/8 female) were enrolled and completed the study (mean age, 30 y; mean weight, 73 kg). HCA was not detected in any sample. DEX suppression resulted in pre-dose HC plasma concentrations (Cp) < 11 ng/mL in all participants; however, in 13 sessions, HC Cp increased at the 24-hour sample (and in 2 sessions at 16 hours), presumably due to dissipation of DEX suppression. As a result, AUC0-∞ could be assessed in only 9 participants. The mean maximum Cp (Cmax) was 194.0 ng/mL for HCA and 392.5 ng/mL for HC (Table). The mean area under the Cp vs time curve from pre-dose to the time of the last quantifiable concentration (AUC0-t) was 3125.0 h·ng/mL for HCA and 3611.0 h·ng/mL for HC. Mean HCA:HC ratios for Cmax and AUC0-t were 0.50 and 0.86, respectively. Cp peaks occurred at 8.0 h and 2.5 h (median) for HCA and HC, respectively (P< 0.0001; Figure). Half-life was 11.7 h for HCA and 5.7 h for HC. Four adverse events (AEs), all mild, were reported; no AEs were serious.
Discussion: The novel, investigational 90 mg HCA suppository administered using the proprietary FDA-cleared applicator demonstrated rapid drug release, sustained HC Cp, and was well tolerated. The unique release and absorption profile of the investigational 90 mg HCA suppository warrants further clinical development for management of inflammatory bowel disease.

Figure: Table. HC Pharmacokinetic Parameters in Plasma

Figure: Figure. Baseline-Corrected Plasma HC Concentration Over Time for HCA Suppository Versus HC Liquid Enema
Disclosures:
Mark Ensign: Cristcot – Employee.
Raj Devarajan: Cristcot – Consultant.
Jennifer Davagian: Cristcot – Employee, Equity.
Mark Ensign, 1, Raj Devarajan, MD2, Jennifer Davagian, 1. P5390 - Pharmacokinetics and Relative Bioavailability of an Investigational Hydrocortisone Acetate Suppository Administered With a Novel FDA-Cleared Applicator Versus Hydrocortisone Liquid Enema, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
