Tuesday Poster Session
Category: IBD
P5382 - Advanced Combination Therapy of Upadacitinib and Biologic Therapy in Refractory Inflammatory Bowel Disease Patients
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- WA
Wafa Alkarni, MBBS (she/her/hers)
King Faisal Specialist Hospital and Research Centre
Riyadh, Ar Riyad, Saudi Arabia
Presenting Author(s)
Wafa Alkarni, MBBS1, Hend Almuhaya, MBBS1, Naif Alrossais, MBBS1, Sameer Desai, 1, Abdulelah Almutairdi, MD1, Badr Al Bawardy, MD2, Mashary Attamimi, MD1
1King Faisal Specialist Hospital and Research Centre, Riyadh, Ar Riyad, Saudi Arabia; 2Yale New Haven Hospital, New Haven, USA; King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, New Haven, CT
Introduction: Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), has increased globally in recent decades. Despite therapeutic advances, remission rates remain modest (30–40% at 12 months). This study aims to assess the safety and efficacy of advanced combination therapy (ACT) using upadacitinib and another biologic in patients with IBD.
Methods: This is a retrospective, single centre study that included patients ≥14 years with confirmed IBD [CD, UC, IBD-unclassified (IBD-U)] who received upadacitinib plus another biologic for ≥4 months. Eligible patients had refractory IBD, defined as failure of ≥2 therapies with different mechanisms of action and active disease clinically or endoscopically. Primary outcome: clinical remission at 4 months. Secondary outcomes: clinical/endoscopic response, endoscopic remission during follow-up, and adverse events during treatment.
Results: A total of 14 patients with refractory IBD were started on ACT (n=10 females [71%]; n=10 with CD [71%]), with a median disease duration of 7 years and a median follow-up of 8 months. All CD patients had Ilecolonic involvement, penetrating disease was present in (n=6; 60%), and (n=8; 80%) had perianal disease. Among UC patients (n=4), 2 (50%) had pancolitis and 2 (50%) had left-sided colitis. The median number of prior advanced therapies was two. At baseline, 62% had elevated C-reactive protein (CRP) and 93% had elevated fecal calprotectin (FCP). Endoscopic assessment revealed active disease in all patients (Table 1). Ustekinumab was the most commonly used concurrent biologic with upadacitinib (n=8; 57%), followed by vedolizumab (n=4; 29%) and risankizumab (n=2; 14%). Upadacitinib was initiated at a dose of 45 mg in most patients (n=13; 93%); (n=5; 36%) received a maintenance dose of 30 mg, while the remaining continued on 45 mg. At 4 months, (n=5; 36%) achieved clinical remission, and (n=6; 43%) showed a clinical response. Endoscopic data were available for 8 patients: (n=2; 25%) achieved remission, (n=2; 25%) showed a response, and (n=4; 50%) had no endoscopic improvement (Figure 1). Two patients (n=2; 14%) developed non-serious infections during ACT. One UC patient (7%) underwent colectomy for uncontrolled disease.
Discussion: ACT with upadacitinib and another biologic demonstrated promising clinical and endoscopic outcomes in refractory IBD and appeared to be well tolerated. Larger studies with longer follow-up duration are needed to further elucidate its long-term efficacy and safety.
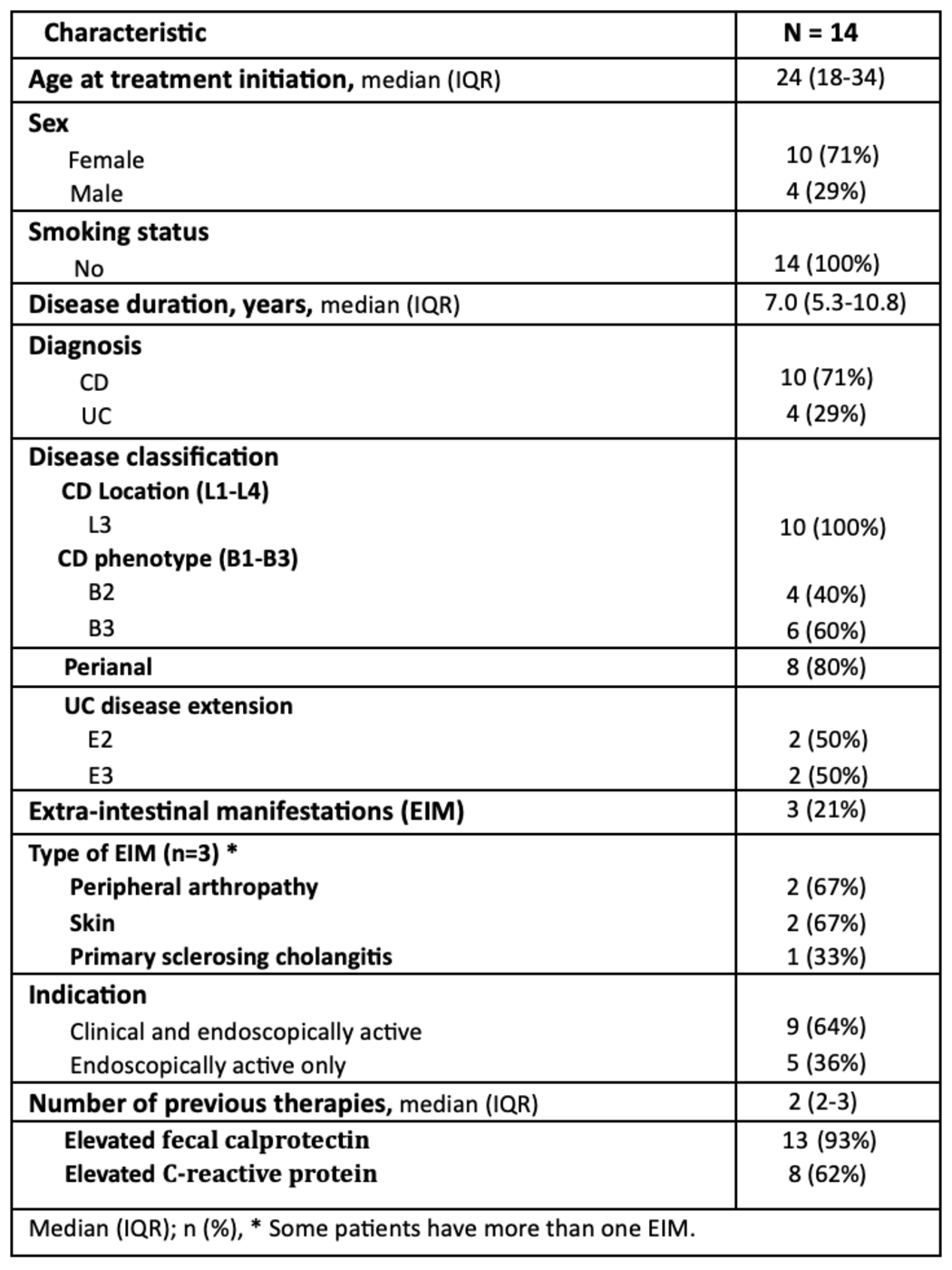
Figure: Table 1: Demographics and other clinical characteristics
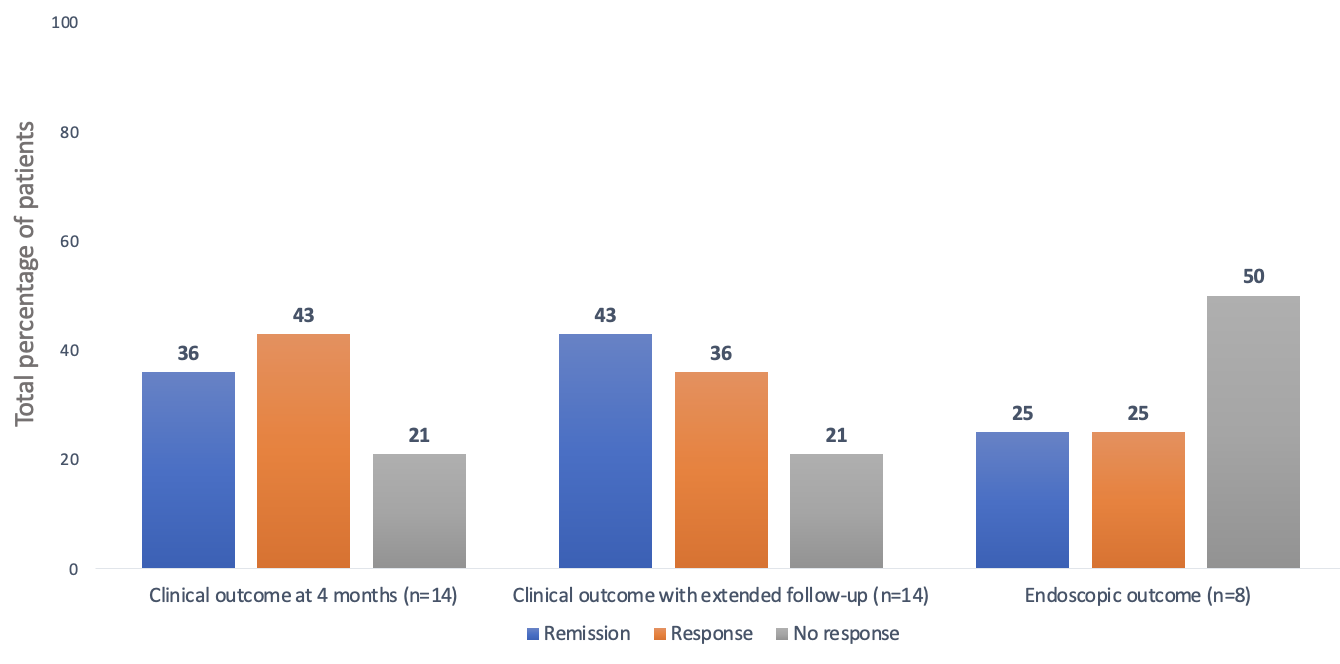
Figure: Figure 1: Clinical and endoscopic outcomes
Disclosures:
Wafa Alkarni indicated no relevant financial relationships.
Hend Almuhaya indicated no relevant financial relationships.
Naif Alrossais indicated no relevant financial relationships.
Sameer Desai indicated no relevant financial relationships.
Abdulelah Almutairdi: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Amgen – Advisory Committee/Board Member. Bristol myers squibb – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Lilly – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Badr Al Bawardy: AbbVie – Advisor or Review Panel Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Hikma – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member. TAKEDA – Advisor or Review Panel Member, Speakers Bureau.
Mashary Attamimi: AbbVie – Speakers Bureau. Hikma – Speakers Bureau. Janssen – Speakers Bureau. Lilly – Speakers Bureau. Pfizer – Speakers Bureau. Sandoz – Speakers Bureau. Takeda – Speakers Bureau.
Wafa Alkarni, MBBS1, Hend Almuhaya, MBBS1, Naif Alrossais, MBBS1, Sameer Desai, 1, Abdulelah Almutairdi, MD1, Badr Al Bawardy, MD2, Mashary Attamimi, MD1. P5382 - Advanced Combination Therapy of Upadacitinib and Biologic Therapy in Refractory Inflammatory Bowel Disease Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1King Faisal Specialist Hospital and Research Centre, Riyadh, Ar Riyad, Saudi Arabia; 2Yale New Haven Hospital, New Haven, USA; King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, New Haven, CT
Introduction: Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), has increased globally in recent decades. Despite therapeutic advances, remission rates remain modest (30–40% at 12 months). This study aims to assess the safety and efficacy of advanced combination therapy (ACT) using upadacitinib and another biologic in patients with IBD.
Methods: This is a retrospective, single centre study that included patients ≥14 years with confirmed IBD [CD, UC, IBD-unclassified (IBD-U)] who received upadacitinib plus another biologic for ≥4 months. Eligible patients had refractory IBD, defined as failure of ≥2 therapies with different mechanisms of action and active disease clinically or endoscopically. Primary outcome: clinical remission at 4 months. Secondary outcomes: clinical/endoscopic response, endoscopic remission during follow-up, and adverse events during treatment.
Results: A total of 14 patients with refractory IBD were started on ACT (n=10 females [71%]; n=10 with CD [71%]), with a median disease duration of 7 years and a median follow-up of 8 months. All CD patients had Ilecolonic involvement, penetrating disease was present in (n=6; 60%), and (n=8; 80%) had perianal disease. Among UC patients (n=4), 2 (50%) had pancolitis and 2 (50%) had left-sided colitis. The median number of prior advanced therapies was two. At baseline, 62% had elevated C-reactive protein (CRP) and 93% had elevated fecal calprotectin (FCP). Endoscopic assessment revealed active disease in all patients (Table 1). Ustekinumab was the most commonly used concurrent biologic with upadacitinib (n=8; 57%), followed by vedolizumab (n=4; 29%) and risankizumab (n=2; 14%). Upadacitinib was initiated at a dose of 45 mg in most patients (n=13; 93%); (n=5; 36%) received a maintenance dose of 30 mg, while the remaining continued on 45 mg. At 4 months, (n=5; 36%) achieved clinical remission, and (n=6; 43%) showed a clinical response. Endoscopic data were available for 8 patients: (n=2; 25%) achieved remission, (n=2; 25%) showed a response, and (n=4; 50%) had no endoscopic improvement (Figure 1). Two patients (n=2; 14%) developed non-serious infections during ACT. One UC patient (7%) underwent colectomy for uncontrolled disease.
Discussion: ACT with upadacitinib and another biologic demonstrated promising clinical and endoscopic outcomes in refractory IBD and appeared to be well tolerated. Larger studies with longer follow-up duration are needed to further elucidate its long-term efficacy and safety.

Figure: Table 1: Demographics and other clinical characteristics

Figure: Figure 1: Clinical and endoscopic outcomes
Disclosures:
Wafa Alkarni indicated no relevant financial relationships.
Hend Almuhaya indicated no relevant financial relationships.
Naif Alrossais indicated no relevant financial relationships.
Sameer Desai indicated no relevant financial relationships.
Abdulelah Almutairdi: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Amgen – Advisory Committee/Board Member. Bristol myers squibb – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Lilly – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Badr Al Bawardy: AbbVie – Advisor or Review Panel Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Hikma – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member. TAKEDA – Advisor or Review Panel Member, Speakers Bureau.
Mashary Attamimi: AbbVie – Speakers Bureau. Hikma – Speakers Bureau. Janssen – Speakers Bureau. Lilly – Speakers Bureau. Pfizer – Speakers Bureau. Sandoz – Speakers Bureau. Takeda – Speakers Bureau.
Wafa Alkarni, MBBS1, Hend Almuhaya, MBBS1, Naif Alrossais, MBBS1, Sameer Desai, 1, Abdulelah Almutairdi, MD1, Badr Al Bawardy, MD2, Mashary Attamimi, MD1. P5382 - Advanced Combination Therapy of Upadacitinib and Biologic Therapy in Refractory Inflammatory Bowel Disease Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
