Tuesday Poster Session
Category: IBD
P5380 - Bowel Urgency Outcomes Are Associated With Clinical Outcomes, Health-Related Quality of Life and Biomarkers in the Etrasimod ELEVATE UC 52 Clinical Trial
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- CC
Christina Cognata, PharmD, MBA
Pfizer Inc
Collegeville, Pennsylvania
Presenting Author(s)
Marla C. Dubinsky, MD1, Erica R. Cohen, MD2, Peter M. Irving, MD3, Martina Goetsch, MD4, Krisztina Lazin, MD4, Abhishek Bhattacharjee, PhD5, Gokul Pradeep, PhD5, Peter Hur, PharmD6, Christina Cognata, PharmD, MBA7, Karolina Wosik, MSc, PhD8, Remo Panaccione, MD9
1Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Capital Digestive Care, Chevy Chase, Maryland, MD, USA, Chevy Chase, MD; 3IBD Unit, Guy’s and St Thomas’ Hospital, London, UK, London, England, United Kingdom; 4Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 5Pfizer Healthcare India Private Ltd, Chennai, India, Chennai, Tamil Nadu, India; 6Pfizer Inc, New York, NY; 7Pfizer Inc, Collegeville, PA; 8Pfizer Canada, Kirkland, PQ, Canada; 9Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1 phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). Etrasimod 2 mg QD improved bowel urgency (BU) at Weeks (Wks) 12 and 52 in the ELEVATE UC clinical program.1
Methods: We assessed associations between BU and efficacy endpoints, health-related quality of life (HRQoL) and biomarkers in patients receiving etrasimod in ELEVATE UC 52 (NCT03945188). BU was assessed at baseline (BL), Wk 12 and Wk 52 via a patient-reported, 11-point Urgency Numerical Rating Scale (NRS; 0–10; none to worst possible BU). We defined patient subgroups as with/without BU remission (NRS ≤ 1) or clinically meaningful improvement (CMI) in BU (NRS ≥ 3-point decrease from BL) at Wks 12 and 52. Associations were assessed between BU remission or CMI in BU (yes/no) and binary efficacy endpoints (multivariable logistic regression), change from BL in HRQoL using the inflammatory bowel diseases questionnaire total score and 36-item short form survey physical and mental component scores (multivariable linear regression) and fecal calprotectin (fCAL; Wilcoxon Rank test) at Wks 12 and 52.
Results: A larger proportion of patients with BU remission (73.6%) and CMI in BU (78.9%) at Wk 12 were naïve to biologic/Janus kinase inhibitors vs experienced. At Wk 12, significantly more patients with vs without BU remission met efficacy endpoints (odds ratio, OR [95% confidence interval, CI]: clinical remission 4.0 [2.3, 7.1]; endoscopic improvement 3.6 [2.1, 6.2]; both p</em> < 0.0001) and had significantly greater improvement in HRQoL scores and lower fCAL (all p < 0.05; Table). A similar association was seen for CMI in BU at Wk 12 (OR [95% CI]: clinical remission 3.7 [2.1, 6.4]; endoscopic improvement 2.6 [1.6, 4.3]; both p < 0.001; Table) and for patients meeting BU outcomes at Wk 52.
Discussion: Bowel urgency outcomes were associated with improvements in clinical and endoscopic endpoints, HRQoL scores and reduced fCAL at Wks 12 and 52. With etrasimod, BU outcomes may be surrogate markers of disease activity and HRQoL improvement.
Reference:
1. Dubinsky MC et al. Am J Gastroenterol 2023; 118: S890–S891.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
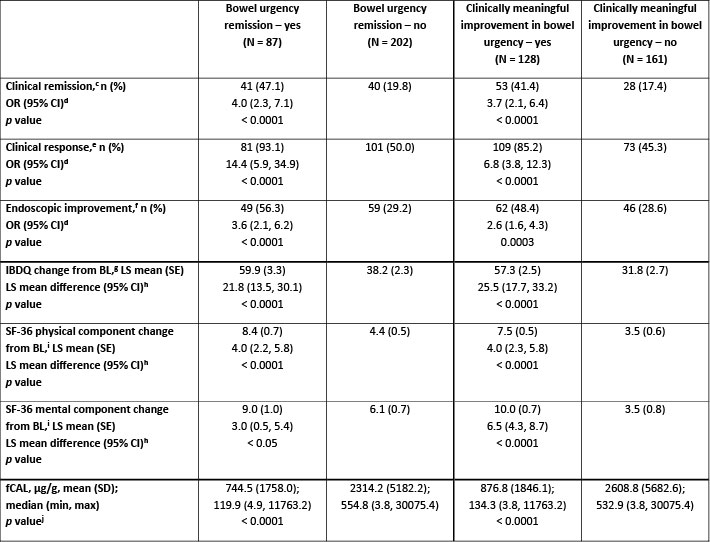
Figure: Table. Association of bowel urgency remission[a] and clinically meaningful improvement in bowel urgency[b] at Wk 12 with clinical and endoscopic endpoints, HRQoL, and fCAL at Wk 12 in ELEVATE UC 52.
Data are presented for the full analysis set (baseline MMS 4–9).
[a]Defined as NRS ≤ 1.
[b]Defined as NRS ≥ 3-point decrease from BL.
[c]Defined as SF subscore = 0 (or = 1 with a ≥ 1-point decrease from BL), RB subscore = 0, and ES ≤ 1 (excluding friability).
[d]OR (95% CI) and p values were obtained from a multivariable logistic regression with a covariate for bowel urgency status (yes/no) at Wk 12, naïve to biologic/Janus kinase inhibitor therapy at BL (yes/no), BL corticosteroid use (yes/no), and BL disease activity (MMS 4–6 or MMS 7–9). Missing responses are considered as nonresponse.
[e]Defined as a ≥ 2-point and ≥ 30% decrease from BL in MMS and a ≥ 1-point decrease from BL in RB subscore, or an absolute RB subscore ≤ 1.
[f]Defined as ES of ≤ 1.
[g]Each response to a total of 32 questions is graded 1–7, with an overall possible score range of 32–224 (very poor to perfect HRQoL).
[h]BL is the last measurement taken prior to the first dose of study treatment. LS mean difference (95% CI) estimates are obtained from a multivariable linear regression model, adjusting for bowel urgency subgroup (yes/no) at Wk 12, BL score of respective endpoint, SF at Wk 12, and RB at Wk 12.
[i]SF-36 comprises eight multi-question domains, each scored 0–100 (worst to best HRQoL) then aggregated into the physical and mental component summary scores.
[j]Wilcoxon test p value is calculated based on normal approximation. Missing values are imputed using the last observation carried forward method.
BL, baseline; CI, confidence interval; ES, endoscopic subscore; fCAL, fecal calprotectin; HRQoL, health-related quality of life; IBDQ, inflammatory bowel disease questionnaire; LS, least squares; max, maximum; min, minimum; MMS, modified Mayo score; N, total number of patients; n, number of responders or patients with observations; NRS, Numerical Rating Scale; OR, odds ratio; RB, rectal bleeding; SD, standard deviation; SE, standard error; SF-36, 36-item short form survey; SF, stool frequency; UC, ulcerative colitis; Wk, Week.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Erica Cohen: AbbVie – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Janssen – Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant. PRIME Education – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Peter Irving: Arena – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisor or Review Panel Member. Gilead – Advisor or Review Panel Member, Speakers Bureau. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. MSD – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Pharmacosmos – Advisor or Review Panel Member. Prometheus – Advisor or Review Panel Member. Roche – Advisor or Review Panel Member. Samsung Bioepis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. Topivert – Advisor or Review Panel Member. VH2 – Advisor or Review Panel Member. Vifor Pharma – Advisor or Review Panel Member. Warner Chilcott – Advisor or Review Panel Member, Speakers Bureau.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Abhishek Bhattacharjee: Pfizer Healthcare India Private Ltd. – Employee. Pfizer Inc – Stock Options.
Gokul Pradeep: Pfizer Healthcare India Private Ltd. – Employee.
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Christina Cognata: Pfizer Inc – Employee, Stock Options.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Marla C. Dubinsky, MD1, Erica R. Cohen, MD2, Peter M. Irving, MD3, Martina Goetsch, MD4, Krisztina Lazin, MD4, Abhishek Bhattacharjee, PhD5, Gokul Pradeep, PhD5, Peter Hur, PharmD6, Christina Cognata, PharmD, MBA7, Karolina Wosik, MSc, PhD8, Remo Panaccione, MD9. P5380 - Bowel Urgency Outcomes Are Associated With Clinical Outcomes, Health-Related Quality of Life and Biomarkers in the Etrasimod ELEVATE UC 52 Clinical Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Capital Digestive Care, Chevy Chase, Maryland, MD, USA, Chevy Chase, MD; 3IBD Unit, Guy’s and St Thomas’ Hospital, London, UK, London, England, United Kingdom; 4Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 5Pfizer Healthcare India Private Ltd, Chennai, India, Chennai, Tamil Nadu, India; 6Pfizer Inc, New York, NY; 7Pfizer Inc, Collegeville, PA; 8Pfizer Canada, Kirkland, PQ, Canada; 9Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1 phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). Etrasimod 2 mg QD improved bowel urgency (BU) at Weeks (Wks) 12 and 52 in the ELEVATE UC clinical program.1
Methods: We assessed associations between BU and efficacy endpoints, health-related quality of life (HRQoL) and biomarkers in patients receiving etrasimod in ELEVATE UC 52 (NCT03945188). BU was assessed at baseline (BL), Wk 12 and Wk 52 via a patient-reported, 11-point Urgency Numerical Rating Scale (NRS; 0–10; none to worst possible BU). We defined patient subgroups as with/without BU remission (NRS ≤ 1) or clinically meaningful improvement (CMI) in BU (NRS ≥ 3-point decrease from BL) at Wks 12 and 52. Associations were assessed between BU remission or CMI in BU (yes/no) and binary efficacy endpoints (multivariable logistic regression), change from BL in HRQoL using the inflammatory bowel diseases questionnaire total score and 36-item short form survey physical and mental component scores (multivariable linear regression) and fecal calprotectin (fCAL; Wilcoxon Rank test) at Wks 12 and 52.
Results: A larger proportion of patients with BU remission (73.6%) and CMI in BU (78.9%) at Wk 12 were naïve to biologic/Janus kinase inhibitors vs experienced. At Wk 12, significantly more patients with vs without BU remission met efficacy endpoints (odds ratio, OR [95% confidence interval, CI]: clinical remission 4.0 [2.3, 7.1]; endoscopic improvement 3.6 [2.1, 6.2]; both p</em> < 0.0001) and had significantly greater improvement in HRQoL scores and lower fCAL (all p < 0.05; Table). A similar association was seen for CMI in BU at Wk 12 (OR [95% CI]: clinical remission 3.7 [2.1, 6.4]; endoscopic improvement 2.6 [1.6, 4.3]; both p < 0.001; Table) and for patients meeting BU outcomes at Wk 52.
Discussion: Bowel urgency outcomes were associated with improvements in clinical and endoscopic endpoints, HRQoL scores and reduced fCAL at Wks 12 and 52. With etrasimod, BU outcomes may be surrogate markers of disease activity and HRQoL improvement.
Reference:
1. Dubinsky MC et al. Am J Gastroenterol 2023; 118: S890–S891.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.

Figure: Table. Association of bowel urgency remission[a] and clinically meaningful improvement in bowel urgency[b] at Wk 12 with clinical and endoscopic endpoints, HRQoL, and fCAL at Wk 12 in ELEVATE UC 52.
Data are presented for the full analysis set (baseline MMS 4–9).
[a]Defined as NRS ≤ 1.
[b]Defined as NRS ≥ 3-point decrease from BL.
[c]Defined as SF subscore = 0 (or = 1 with a ≥ 1-point decrease from BL), RB subscore = 0, and ES ≤ 1 (excluding friability).
[d]OR (95% CI) and p values were obtained from a multivariable logistic regression with a covariate for bowel urgency status (yes/no) at Wk 12, naïve to biologic/Janus kinase inhibitor therapy at BL (yes/no), BL corticosteroid use (yes/no), and BL disease activity (MMS 4–6 or MMS 7–9). Missing responses are considered as nonresponse.
[e]Defined as a ≥ 2-point and ≥ 30% decrease from BL in MMS and a ≥ 1-point decrease from BL in RB subscore, or an absolute RB subscore ≤ 1.
[f]Defined as ES of ≤ 1.
[g]Each response to a total of 32 questions is graded 1–7, with an overall possible score range of 32–224 (very poor to perfect HRQoL).
[h]BL is the last measurement taken prior to the first dose of study treatment. LS mean difference (95% CI) estimates are obtained from a multivariable linear regression model, adjusting for bowel urgency subgroup (yes/no) at Wk 12, BL score of respective endpoint, SF at Wk 12, and RB at Wk 12.
[i]SF-36 comprises eight multi-question domains, each scored 0–100 (worst to best HRQoL) then aggregated into the physical and mental component summary scores.
[j]Wilcoxon test p value is calculated based on normal approximation. Missing values are imputed using the last observation carried forward method.
BL, baseline; CI, confidence interval; ES, endoscopic subscore; fCAL, fecal calprotectin; HRQoL, health-related quality of life; IBDQ, inflammatory bowel disease questionnaire; LS, least squares; max, maximum; min, minimum; MMS, modified Mayo score; N, total number of patients; n, number of responders or patients with observations; NRS, Numerical Rating Scale; OR, odds ratio; RB, rectal bleeding; SD, standard deviation; SE, standard error; SF-36, 36-item short form survey; SF, stool frequency; UC, ulcerative colitis; Wk, Week.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Erica Cohen: AbbVie – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Janssen – Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant. PRIME Education – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Peter Irving: Arena – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisor or Review Panel Member. Gilead – Advisor or Review Panel Member, Speakers Bureau. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. MSD – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Pharmacosmos – Advisor or Review Panel Member. Prometheus – Advisor or Review Panel Member. Roche – Advisor or Review Panel Member. Samsung Bioepis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. Topivert – Advisor or Review Panel Member. VH2 – Advisor or Review Panel Member. Vifor Pharma – Advisor or Review Panel Member. Warner Chilcott – Advisor or Review Panel Member, Speakers Bureau.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Abhishek Bhattacharjee: Pfizer Healthcare India Private Ltd. – Employee. Pfizer Inc – Stock Options.
Gokul Pradeep: Pfizer Healthcare India Private Ltd. – Employee.
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Christina Cognata: Pfizer Inc – Employee, Stock Options.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Marla C. Dubinsky, MD1, Erica R. Cohen, MD2, Peter M. Irving, MD3, Martina Goetsch, MD4, Krisztina Lazin, MD4, Abhishek Bhattacharjee, PhD5, Gokul Pradeep, PhD5, Peter Hur, PharmD6, Christina Cognata, PharmD, MBA7, Karolina Wosik, MSc, PhD8, Remo Panaccione, MD9. P5380 - Bowel Urgency Outcomes Are Associated With Clinical Outcomes, Health-Related Quality of Life and Biomarkers in the Etrasimod ELEVATE UC 52 Clinical Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
