Tuesday Poster Session
Category: IBD
P5374 - Safety of Botulinum Toxin in Patients on Tumor Necrosis Factor Inhibitors: A Retrospective Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Helene Bloom, DO
Allegheny Health Network Medicine Institute
Pittsburgh, PA
Presenting Author(s)
Helene Bloom, DO1, Christina Dimaria, DO, RD2, Heitham Abdul-Baki, MD2
1Allegheny Health Network Medicine Institute, Pittsburgh, PA; 2Allegheny Health Network, Pittsburgh, PA
Introduction: With the prevalence of autoimmune diseases such as inflammatory bowel disease, ankylosing spondylitis and rheumatoid arthritis (RA) increasing, the number of patients maintained on anti-tumor necrosis factors (anti-TNFs) is also rising. As many of these diseases are chronic, patients often require lifelong maintenance on anti-TNFs. During their lifetime, they may also develop other diseases, conditions, or cosmetic reasons requiring the use of botulinum toxin (Botox). The use of Botox has been employed in the treatment of many conditions across multiple medical subspecialties; however, there has been limited investigation into the use of Botox in patients treated with anti-TNFs. As safety and efficacy have not been established in this population, it is difficult to provide evidenced-based recommendations. This study aims to evaluate the safety of Botox in this subset of patients.
Methods: This retrospective study utilized the electronic medical record identifying patients > 18 years who were on anti-TNFs while receiving Botox injections. 45 patients were identified, and 15 met the criteria for inclusion. The indication for Botox administration, side effects of injection, presence of an immune disease flare or a change in their current anti-TNF dose or medication following injection were studied.
Results: While only 15 patients met inclusion criteria, a total of 41 Botox injections were studied longitudinally over the course of one year. Patients were on anti-TNF medications for multiple diseases including ankylosing spondylitis, Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, RA, or a combination of these. As for the diagnosis for Botox, most patients had migraines (10, 66.67%). Of the 15 patients, 93.33% (14/15) had improvement in their symptoms with their first dose and 100% experienced improvement with a subsequent dose. Only 1 (6.67%) experienced injection site pain. Other mild side effects included dizziness, hoarseness and weakness, although these did not deter further Botox administration. Only one patient receiving treatment for Chron’s Disease required an escalation of dosage in their anti-TNF. After review, it was identified that this was related to the progression of their disease rather than the injection itself.
Discussion: Overall, the use of Botox was well tolerated with minimal adverse reactions in patients receiving anti-TNFs. There were limited side effects and no changes in the dose or biologic therapy as a result of the administration of Botox.
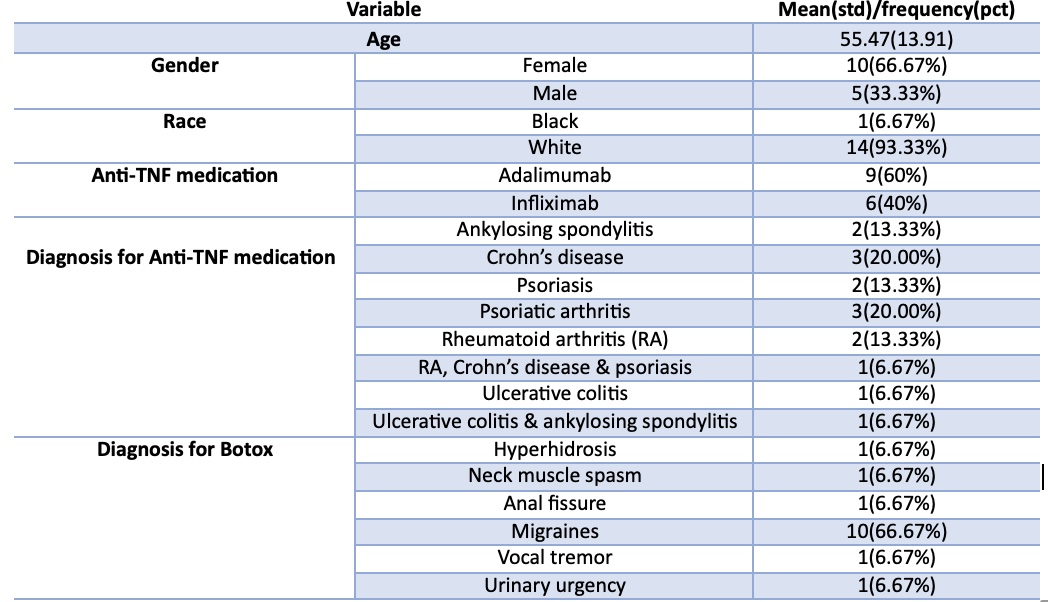
Figure: Descriptive Statistics of Demographics
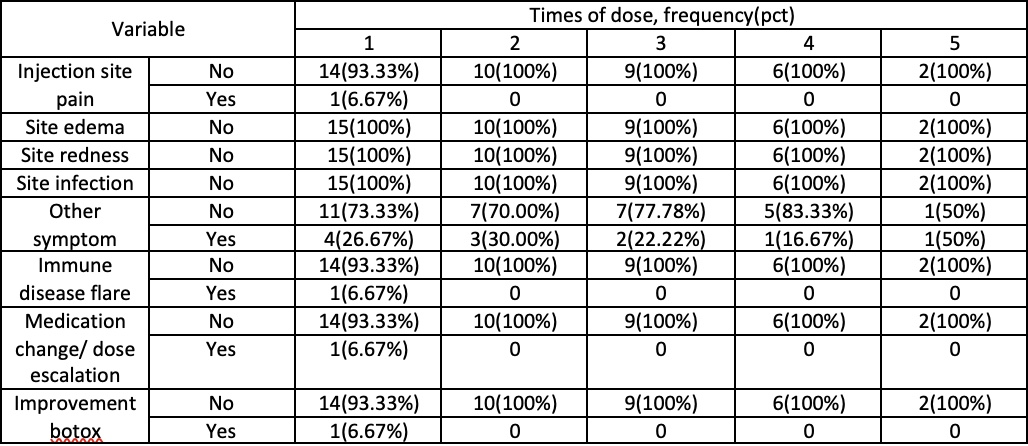
Figure: Descriptive Statistics for Adverse Outcomes at each dose
Disclosures:
Helene Bloom indicated no relevant financial relationships.
Christina Dimaria indicated no relevant financial relationships.
Heitham Abdul-Baki: Abbvie – Speakers Bureau. Takeda – Speakers Bureau.
Helene Bloom, DO1, Christina Dimaria, DO, RD2, Heitham Abdul-Baki, MD2. P5374 - Safety of Botulinum Toxin in Patients on Tumor Necrosis Factor Inhibitors: A Retrospective Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny Health Network Medicine Institute, Pittsburgh, PA; 2Allegheny Health Network, Pittsburgh, PA
Introduction: With the prevalence of autoimmune diseases such as inflammatory bowel disease, ankylosing spondylitis and rheumatoid arthritis (RA) increasing, the number of patients maintained on anti-tumor necrosis factors (anti-TNFs) is also rising. As many of these diseases are chronic, patients often require lifelong maintenance on anti-TNFs. During their lifetime, they may also develop other diseases, conditions, or cosmetic reasons requiring the use of botulinum toxin (Botox). The use of Botox has been employed in the treatment of many conditions across multiple medical subspecialties; however, there has been limited investigation into the use of Botox in patients treated with anti-TNFs. As safety and efficacy have not been established in this population, it is difficult to provide evidenced-based recommendations. This study aims to evaluate the safety of Botox in this subset of patients.
Methods: This retrospective study utilized the electronic medical record identifying patients > 18 years who were on anti-TNFs while receiving Botox injections. 45 patients were identified, and 15 met the criteria for inclusion. The indication for Botox administration, side effects of injection, presence of an immune disease flare or a change in their current anti-TNF dose or medication following injection were studied.
Results: While only 15 patients met inclusion criteria, a total of 41 Botox injections were studied longitudinally over the course of one year. Patients were on anti-TNF medications for multiple diseases including ankylosing spondylitis, Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, RA, or a combination of these. As for the diagnosis for Botox, most patients had migraines (10, 66.67%). Of the 15 patients, 93.33% (14/15) had improvement in their symptoms with their first dose and 100% experienced improvement with a subsequent dose. Only 1 (6.67%) experienced injection site pain. Other mild side effects included dizziness, hoarseness and weakness, although these did not deter further Botox administration. Only one patient receiving treatment for Chron’s Disease required an escalation of dosage in their anti-TNF. After review, it was identified that this was related to the progression of their disease rather than the injection itself.
Discussion: Overall, the use of Botox was well tolerated with minimal adverse reactions in patients receiving anti-TNFs. There were limited side effects and no changes in the dose or biologic therapy as a result of the administration of Botox.

Figure: Descriptive Statistics of Demographics

Figure: Descriptive Statistics for Adverse Outcomes at each dose
Disclosures:
Helene Bloom indicated no relevant financial relationships.
Christina Dimaria indicated no relevant financial relationships.
Heitham Abdul-Baki: Abbvie – Speakers Bureau. Takeda – Speakers Bureau.
Helene Bloom, DO1, Christina Dimaria, DO, RD2, Heitham Abdul-Baki, MD2. P5374 - Safety of Botulinum Toxin in Patients on Tumor Necrosis Factor Inhibitors: A Retrospective Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
