Tuesday Poster Session
Category: IBD
P5373 - Efficacy of Filgotinib in Patients With Ulcerative Colitis: A Systemic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- VC
Vinay Chandramouli Bellur
ramaiah medical college
Bangalore, Karnataka, India
Presenting Author(s)
Shradha Chervittara Karaveetil, 1, Vinay Chandramouli Bellur, 1, Ananya Prasad, 1, Omar Oudit, DO2, Trisha Chandra Mohan, 3, Rishikesh R. Magaji, 3, Anusha Giri, 1, Aryan Gupta, 4, Keerthi Balaji Babu Naidu, 5, Aashray Alva, 1, Pavan Kumara Kasam Shiva, 4, Ashish Sivaratri, 6, A. Shree Charan, 7, Druvadeep Srinivas, 8, Lakshana Mukundan, 6, Sravani Bhavanam, 9
1ramaiah medical college, Bangalore, Karnataka, India; 2Brookdale University Hospital Medical Center, Brooklyn, NY; 3BGS Global Institute of Medical Sciences, Bangalore, Karnataka, India; 4bangalore medical college and research institute, Bangalore, Karnataka, India; 5Ramaiah medical college, Bangalore, Karnataka, India; 6Bangalore Medical College, Bangalore, Karnataka, India; 7Ramaiah Medical College, Bangalore, Karnataka, India; 8rajarajeshwari medical college & hospital, Bangalore, Karnataka, India; 9Brookdale University Hospital Medical Center, Bangalore, Karnataka, India
Introduction: Filgotinib, a novel JAK1 selective inhibitor, reduces inflammation by disrupting the signaling pathways of immune cells in response to cytokines. It has recently been approved as therapy for moderate-to-severe Ulcerative Colitis (UC). It has improved drug clearance and is effective in both biologic-naïve as well as biologic-failed patients (TNF antagonists, Vedolizumab, etc. used as first-line treatment) and has been studied through clinical trials such as the SELECTION trial. In this review, we assess the efficacy of Filgotinib through the clinical and pathological regression of UC.
Methods: The review conducted follows PRISMA guidelines. PUBMED, Google Scholar and Science-Direct were extensively searched using a search term to retrieve articles. Articles which included the assessment of complete remission of UC following Filgotinib therapy and standard treatment were included.
The data was analysed using the Meta, Metadata and the Metafor packages of R Studio. The proportion of the Odds Ratio (OR) of complete remission of UC following Filgotinib therapy compared to standard care/Placebo was assessed as our primary outcome. The pooled ratio of complete remission of UC following different doses was assessed as secondary outcomes. The Mantel-Haenszel method and the Inverse variance method were utilised to analyse the odds ratio.
Results: The study includes a total of 10 papers with 2710 subjects receiving Filgotinib therapy and 2130 patients under standard care/placebo for UC. The OR of Complete remission of Ulcerative Colitis was not statistically significant in both groups indicating similar clinical efficacy in both groups.{OR=1.31 (0.94;1.82)} , 95% CI , p< 0.01 ,I^2=86%). Pooled proportion of Complete remission of UC was assessed in 2 different drug doses among induction and maintenance phases. Complete remission was achieved in 40% of the population receiving 200mg Filgotinib therapy and 23% in the 100mg Filgotinib therapy group. The risk of adverse events was similar in both the groups indicating similar safety index. (OR = 0.8508(0.6391; 1.144) , I^2=29% , p=0.22)
Discussion:
This review indicates that a higher dose of Filgotinib may lead to a higher probability of clinical regression without any significant rise in the adverse effects. Studies focusing heavily on a lower dose of Filgotinib are still ongoing and its efficacy is yet to be analysed completely, however this review suggests that current dosage of 200mg may be safely continued for the treatment of Ulcerative Colitis.
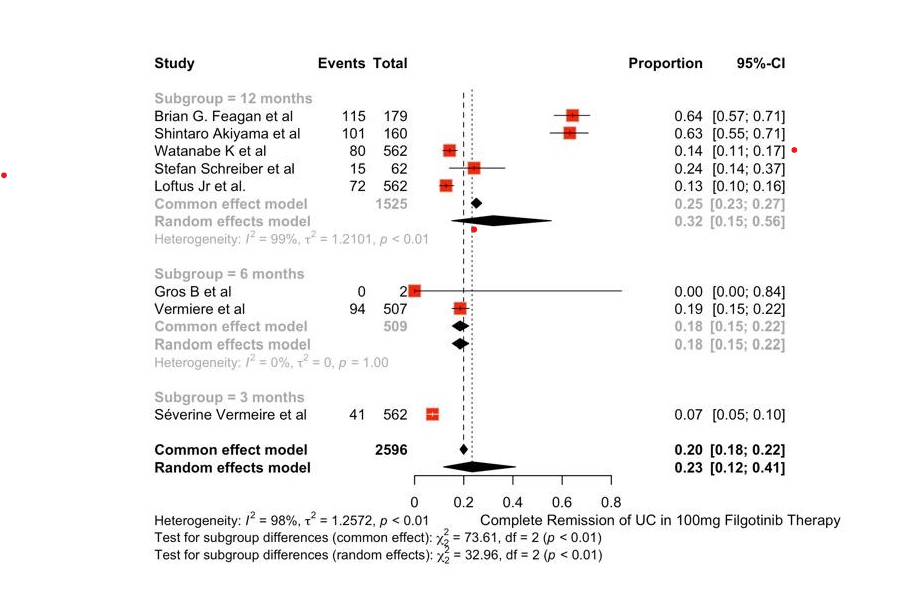
Figure: Complete Remission of UC with Filgotinib 100 mg
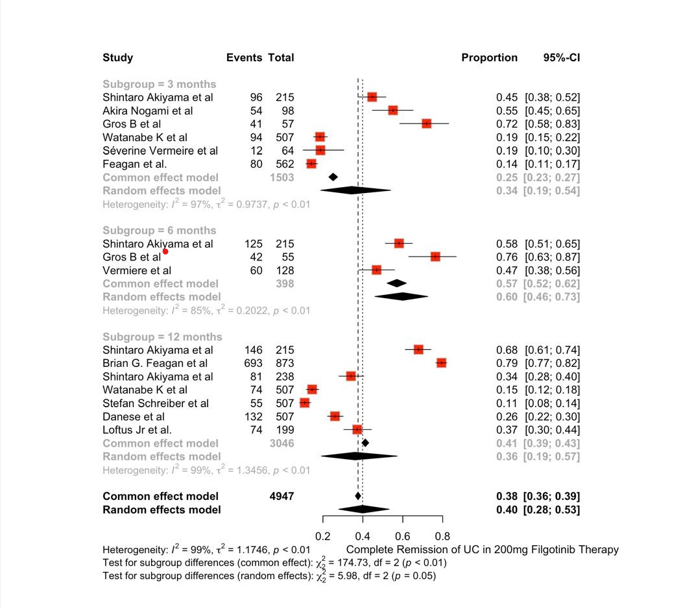
Figure: Complete Remission of UC with Filgotinib 200 mg
Disclosures:
Shradha Chervittara Karaveetil indicated no relevant financial relationships.
Vinay Chandramouli Bellur indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Omar Oudit indicated no relevant financial relationships.
Trisha Chandra Mohan indicated no relevant financial relationships.
Rishikesh R. Magaji indicated no relevant financial relationships.
Anusha Giri indicated no relevant financial relationships.
Aryan Gupta indicated no relevant financial relationships.
Keerthi Balaji Babu Naidu indicated no relevant financial relationships.
Aashray Alva indicated no relevant financial relationships.
Pavan Kumara Kasam Shiva indicated no relevant financial relationships.
Ashish Sivaratri indicated no relevant financial relationships.
A. Shree Charan indicated no relevant financial relationships.
Druvadeep Srinivas indicated no relevant financial relationships.
Lakshana Mukundan indicated no relevant financial relationships.
Sravani Bhavanam indicated no relevant financial relationships.
Shradha Chervittara Karaveetil, 1, Vinay Chandramouli Bellur, 1, Ananya Prasad, 1, Omar Oudit, DO2, Trisha Chandra Mohan, 3, Rishikesh R. Magaji, 3, Anusha Giri, 1, Aryan Gupta, 4, Keerthi Balaji Babu Naidu, 5, Aashray Alva, 1, Pavan Kumara Kasam Shiva, 4, Ashish Sivaratri, 6, A. Shree Charan, 7, Druvadeep Srinivas, 8, Lakshana Mukundan, 6, Sravani Bhavanam, 9. P5373 - Efficacy of Filgotinib in Patients With Ulcerative Colitis: A Systemic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1ramaiah medical college, Bangalore, Karnataka, India; 2Brookdale University Hospital Medical Center, Brooklyn, NY; 3BGS Global Institute of Medical Sciences, Bangalore, Karnataka, India; 4bangalore medical college and research institute, Bangalore, Karnataka, India; 5Ramaiah medical college, Bangalore, Karnataka, India; 6Bangalore Medical College, Bangalore, Karnataka, India; 7Ramaiah Medical College, Bangalore, Karnataka, India; 8rajarajeshwari medical college & hospital, Bangalore, Karnataka, India; 9Brookdale University Hospital Medical Center, Bangalore, Karnataka, India
Introduction: Filgotinib, a novel JAK1 selective inhibitor, reduces inflammation by disrupting the signaling pathways of immune cells in response to cytokines. It has recently been approved as therapy for moderate-to-severe Ulcerative Colitis (UC). It has improved drug clearance and is effective in both biologic-naïve as well as biologic-failed patients (TNF antagonists, Vedolizumab, etc. used as first-line treatment) and has been studied through clinical trials such as the SELECTION trial. In this review, we assess the efficacy of Filgotinib through the clinical and pathological regression of UC.
Methods: The review conducted follows PRISMA guidelines. PUBMED, Google Scholar and Science-Direct were extensively searched using a search term to retrieve articles. Articles which included the assessment of complete remission of UC following Filgotinib therapy and standard treatment were included.
The data was analysed using the Meta, Metadata and the Metafor packages of R Studio. The proportion of the Odds Ratio (OR) of complete remission of UC following Filgotinib therapy compared to standard care/Placebo was assessed as our primary outcome. The pooled ratio of complete remission of UC following different doses was assessed as secondary outcomes. The Mantel-Haenszel method and the Inverse variance method were utilised to analyse the odds ratio.
Results: The study includes a total of 10 papers with 2710 subjects receiving Filgotinib therapy and 2130 patients under standard care/placebo for UC. The OR of Complete remission of Ulcerative Colitis was not statistically significant in both groups indicating similar clinical efficacy in both groups.{OR=1.31 (0.94;1.82)} , 95% CI , p< 0.01 ,I^2=86%). Pooled proportion of Complete remission of UC was assessed in 2 different drug doses among induction and maintenance phases. Complete remission was achieved in 40% of the population receiving 200mg Filgotinib therapy and 23% in the 100mg Filgotinib therapy group. The risk of adverse events was similar in both the groups indicating similar safety index. (OR = 0.8508(0.6391; 1.144) , I^2=29% , p=0.22)
Discussion:
This review indicates that a higher dose of Filgotinib may lead to a higher probability of clinical regression without any significant rise in the adverse effects. Studies focusing heavily on a lower dose of Filgotinib are still ongoing and its efficacy is yet to be analysed completely, however this review suggests that current dosage of 200mg may be safely continued for the treatment of Ulcerative Colitis.

Figure: Complete Remission of UC with Filgotinib 100 mg

Figure: Complete Remission of UC with Filgotinib 200 mg
Disclosures:
Shradha Chervittara Karaveetil indicated no relevant financial relationships.
Vinay Chandramouli Bellur indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Omar Oudit indicated no relevant financial relationships.
Trisha Chandra Mohan indicated no relevant financial relationships.
Rishikesh R. Magaji indicated no relevant financial relationships.
Anusha Giri indicated no relevant financial relationships.
Aryan Gupta indicated no relevant financial relationships.
Keerthi Balaji Babu Naidu indicated no relevant financial relationships.
Aashray Alva indicated no relevant financial relationships.
Pavan Kumara Kasam Shiva indicated no relevant financial relationships.
Ashish Sivaratri indicated no relevant financial relationships.
A. Shree Charan indicated no relevant financial relationships.
Druvadeep Srinivas indicated no relevant financial relationships.
Lakshana Mukundan indicated no relevant financial relationships.
Sravani Bhavanam indicated no relevant financial relationships.
Shradha Chervittara Karaveetil, 1, Vinay Chandramouli Bellur, 1, Ananya Prasad, 1, Omar Oudit, DO2, Trisha Chandra Mohan, 3, Rishikesh R. Magaji, 3, Anusha Giri, 1, Aryan Gupta, 4, Keerthi Balaji Babu Naidu, 5, Aashray Alva, 1, Pavan Kumara Kasam Shiva, 4, Ashish Sivaratri, 6, A. Shree Charan, 7, Druvadeep Srinivas, 8, Lakshana Mukundan, 6, Sravani Bhavanam, 9. P5373 - Efficacy of Filgotinib in Patients With Ulcerative Colitis: A Systemic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
