Tuesday Poster Session
Category: IBD
P5357 - Biologics and Small Molecule Inhibitors for Mucosal Healing in Ulcerative Colitis: A Comparative Meta-Analysis of Endoscopic Outcomes
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Progga P. Kapuria, MBBS2, Majdi Salahie, MD3, Sadia Maisha, MBBS4, Asif Zamir, MD, FACG5
1DHR Health, Edinburg, Tx, McAllen, TX; 2Dhaka Medical College and Hospital, Henrico, VA; 3DHR Health, McAllen, TX; 4Sher-e- Bangla Medical College, Bangladesh, Barisal, Barisal, Bangladesh; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Mucosal healing in ulcerative colitis (UC) is a validated therapeutic goal linked to sustained clinical remission and decreased colectomy rates. Both biologics and small molecule inhibitors (SMIs) are approved for moderate-to-severe UC; however, comparative data regarding their effectiveness in achieving endoscopic healing remain limited and fragmented.
This study aimed to compare the mucosal healing efficacy of biologic therapies and SMIs in UC through a pooled analysis of randomized controlled trials (RCTs), with emphasis on endoscopic endpoints and safety profiles.
Methods: A systematic review and meta-analysis was performed per PRISMA guidelines. Databases searched included PubMed (2020–2025), Cochrane Central (2010–2025), and Google Scholar (2015–2025). RCTs evaluating biologics (n=4) or SMIs (n=3) versus placebo or standard care in adult UC patients were included. The primary outcome was mucosal healing, defined as a Mayo endoscopic subscore ≤1. Secondary outcomes included treatment-related adverse events (AEs). Pooled risk ratios (RR) and 95% confidence intervals (CI) were calculated using random-effects models. Statistical heterogeneity was assessed with the I² statistic.
Results: Seven RCTs comprising 2,103 patients were included.
Figure 1 illustrates the pooled risk ratios for mucosal healing across both therapeutic classes.
Discussion: Biologics and SMIs both confer significant benefits in promoting mucosal healing in UC. No therapeutic class demonstrated clear superiority in efficacy or safety. Clinical decision-making should integrate patient-specific factors such as response kinetics, adverse event risk, and prior treatment exposure. These findings underscore the necessity for direct comparative trials to further refine treatment algorithms in ulcerative colitis.
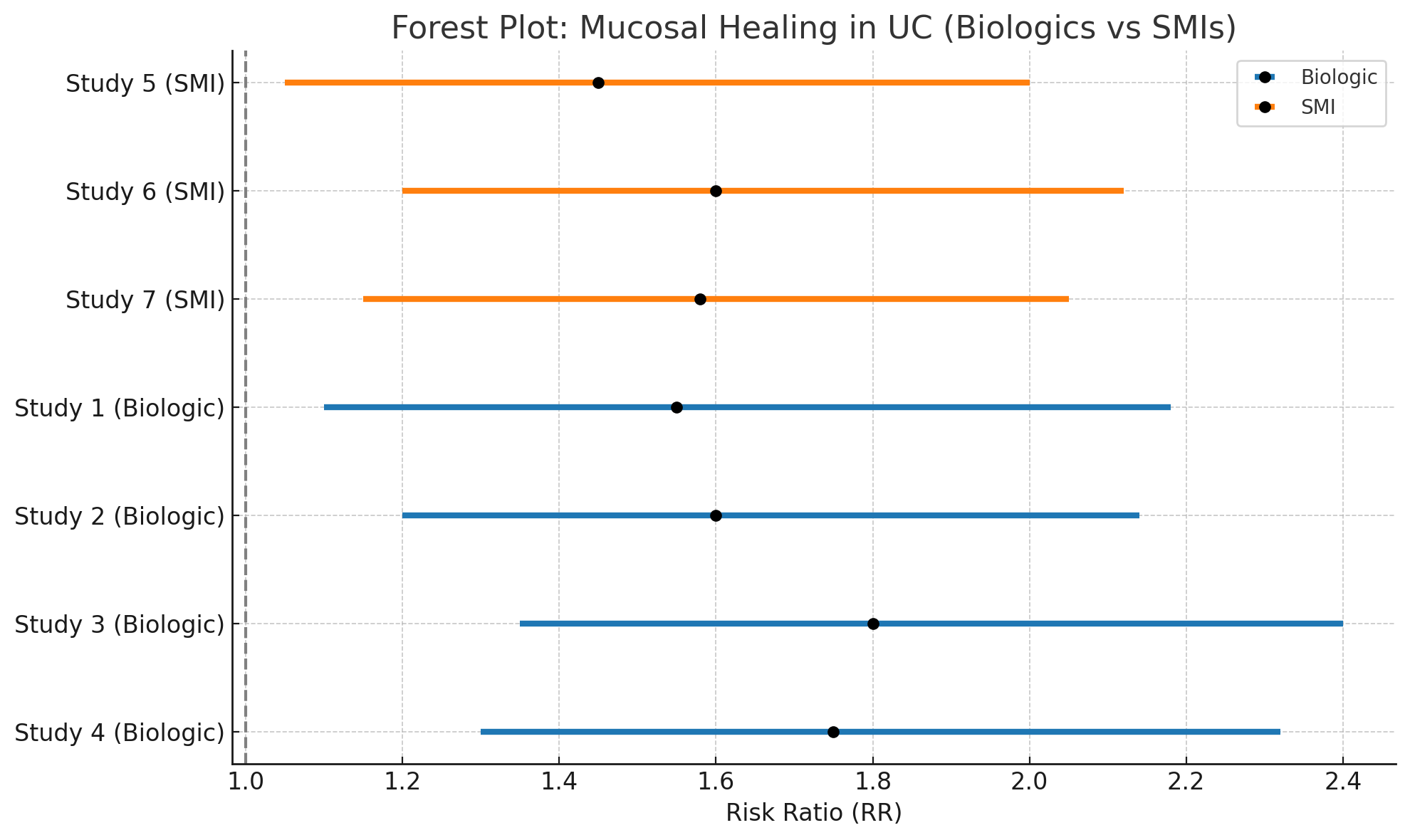
Figure: Figure: Forest Plot: Risk Ratios for Mucosal Healing in Ulcerative Colitis with Biologics and Small Molecule Inhibitors.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Progga Kapuria indicated no relevant financial relationships.
Majdi Salahie indicated no relevant financial relationships.
Sadia Maisha indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Progga P. Kapuria, MBBS2, Majdi Salahie, MD3, Sadia Maisha, MBBS4, Asif Zamir, MD, FACG5. P5357 - Biologics and Small Molecule Inhibitors for Mucosal Healing in Ulcerative Colitis: A Comparative Meta-Analysis of Endoscopic Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2Dhaka Medical College and Hospital, Henrico, VA; 3DHR Health, McAllen, TX; 4Sher-e- Bangla Medical College, Bangladesh, Barisal, Barisal, Bangladesh; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Mucosal healing in ulcerative colitis (UC) is a validated therapeutic goal linked to sustained clinical remission and decreased colectomy rates. Both biologics and small molecule inhibitors (SMIs) are approved for moderate-to-severe UC; however, comparative data regarding their effectiveness in achieving endoscopic healing remain limited and fragmented.
This study aimed to compare the mucosal healing efficacy of biologic therapies and SMIs in UC through a pooled analysis of randomized controlled trials (RCTs), with emphasis on endoscopic endpoints and safety profiles.
Methods: A systematic review and meta-analysis was performed per PRISMA guidelines. Databases searched included PubMed (2020–2025), Cochrane Central (2010–2025), and Google Scholar (2015–2025). RCTs evaluating biologics (n=4) or SMIs (n=3) versus placebo or standard care in adult UC patients were included. The primary outcome was mucosal healing, defined as a Mayo endoscopic subscore ≤1. Secondary outcomes included treatment-related adverse events (AEs). Pooled risk ratios (RR) and 95% confidence intervals (CI) were calculated using random-effects models. Statistical heterogeneity was assessed with the I² statistic.
Results: Seven RCTs comprising 2,103 patients were included.
- Biologics significantly improved mucosal healing compared to control (RR: 1.68; 95% CI: 1.24–2.27; I² = 46%).
- SMIs also showed a significant effect (RR: 1.55; 95% CI: 1.19–2.03; I² = 35%).
- Indirect comparison between biologics and SMIs revealed no significant difference (RR: 1.08; 95% CI: 0.83–1.41).
- Adverse events were comparable between groups (pooled RR: 1.03; 95% CI: 0.89–1.19; I² = 21%).
- Subgroup analysis indicated a more rapid onset of response with SMIs, particularly tofacitinib.
Figure 1 illustrates the pooled risk ratios for mucosal healing across both therapeutic classes.
Discussion: Biologics and SMIs both confer significant benefits in promoting mucosal healing in UC. No therapeutic class demonstrated clear superiority in efficacy or safety. Clinical decision-making should integrate patient-specific factors such as response kinetics, adverse event risk, and prior treatment exposure. These findings underscore the necessity for direct comparative trials to further refine treatment algorithms in ulcerative colitis.

Figure: Figure: Forest Plot: Risk Ratios for Mucosal Healing in Ulcerative Colitis with Biologics and Small Molecule Inhibitors.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Progga Kapuria indicated no relevant financial relationships.
Majdi Salahie indicated no relevant financial relationships.
Sadia Maisha indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Progga P. Kapuria, MBBS2, Majdi Salahie, MD3, Sadia Maisha, MBBS4, Asif Zamir, MD, FACG5. P5357 - Biologics and Small Molecule Inhibitors for Mucosal Healing in Ulcerative Colitis: A Comparative Meta-Analysis of Endoscopic Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
