Tuesday Poster Session
Category: IBD
P5355 - Intermittent Fasting Improves Colitis Outcomes in Preclinical IBD Models: A Meta-Analysis to Guide Human Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Md. Sanjidul Alam, MBBS2, Mahanaz Nabi. Ansa, MBBS3, Nadia Smita, 4, Rishika Trivedi, MD5, Asif Zamir, MD, FACG6
1DHR Health, Edinburg, Tx, McAllen, TX; 2Sylhet MAG Osmani Medical College Hospital, Atlantic City, NJ; 3Sylhet MAG Osmani Medical College Hospital, Sylhet, Sylhet, Bangladesh; 4University of Texas Rio Grande Valley, Edinburg, TX; 5DHR Health, McAllen, TX; 6DHR Health Gastroenterology, Edinburg, TX
Introduction: Inflammatory Bowel Disease (IBD), including Crohn’s disease and ulcerative colitis, is a lifelong disorder marked by immune dysregulation, mucosal injury, and microbiome imbalance. While pharmacologic therapies dominate current treatment algorithms, non-drug strategies like intermittent fasting (IF) remain underexplored. This reflects increasing interest in holistic, low-toxicity adjuncts for IBD. Despite emerging animal data, no consolidated evidence exists to guide translational research. This meta-analysis aimed to evaluate the effects of IF on inflammatory, histologic, microbial, and clinical outcomes in preclinical IBD models and assess its readiness for human trial design.
Methods: Following PRISMA guidelines, we conducted a systematic review and meta-analysis across PubMed, Embase, Cochrane Library, ScienceDirect, Google Scholar, and ClinicalTrials.gov (2000–2024) for preclinical or clinical studies evaluating IF in chemically induced colitis. Inclusion criteria required measurement of histologic inflammation, cytokines (e.g., TNF-α, IL-6), microbiota composition, and/or behavioral indicators. Seven murine studies met criteria. Data extraction was independently performed by two reviewers; pooled standardized mean differences (SMD) and qualitative synthesis were used where feasible.
Results:
Discussion: Intermittent fasting confers reproducible anti-inflammatory, mucosal, and microbial benefits in preclinical IBD models. The absence of human trials highlights the urgent need for clinical evaluation. These findings offer a mechanistically sound, non-pharmacologic rationale to inform first-in-human studies for IBD remission and adjunctive maintenance.
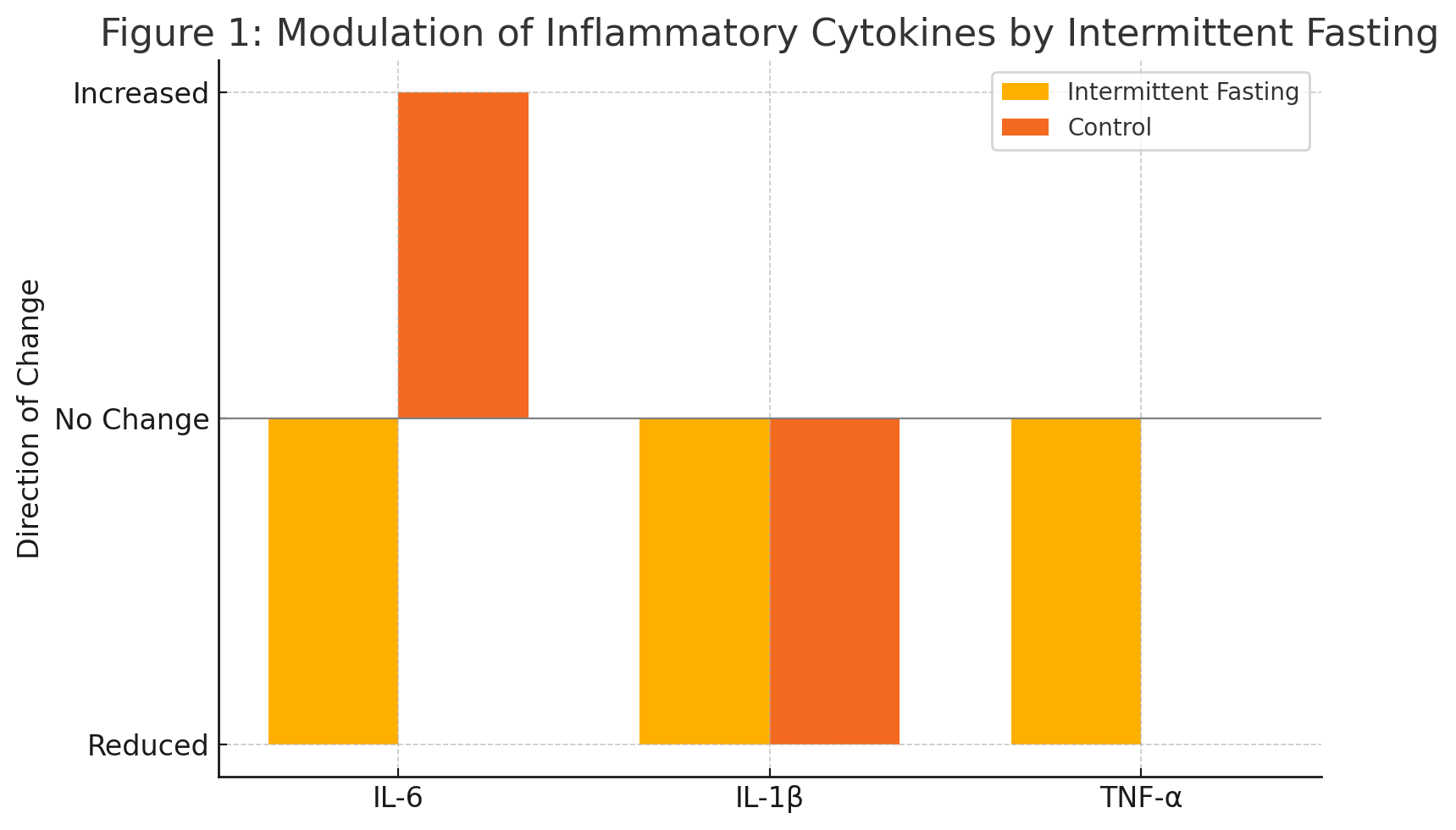
Figure: Figure 1: Modulation of Inflammatory Cytokines by Intermittent Fasting
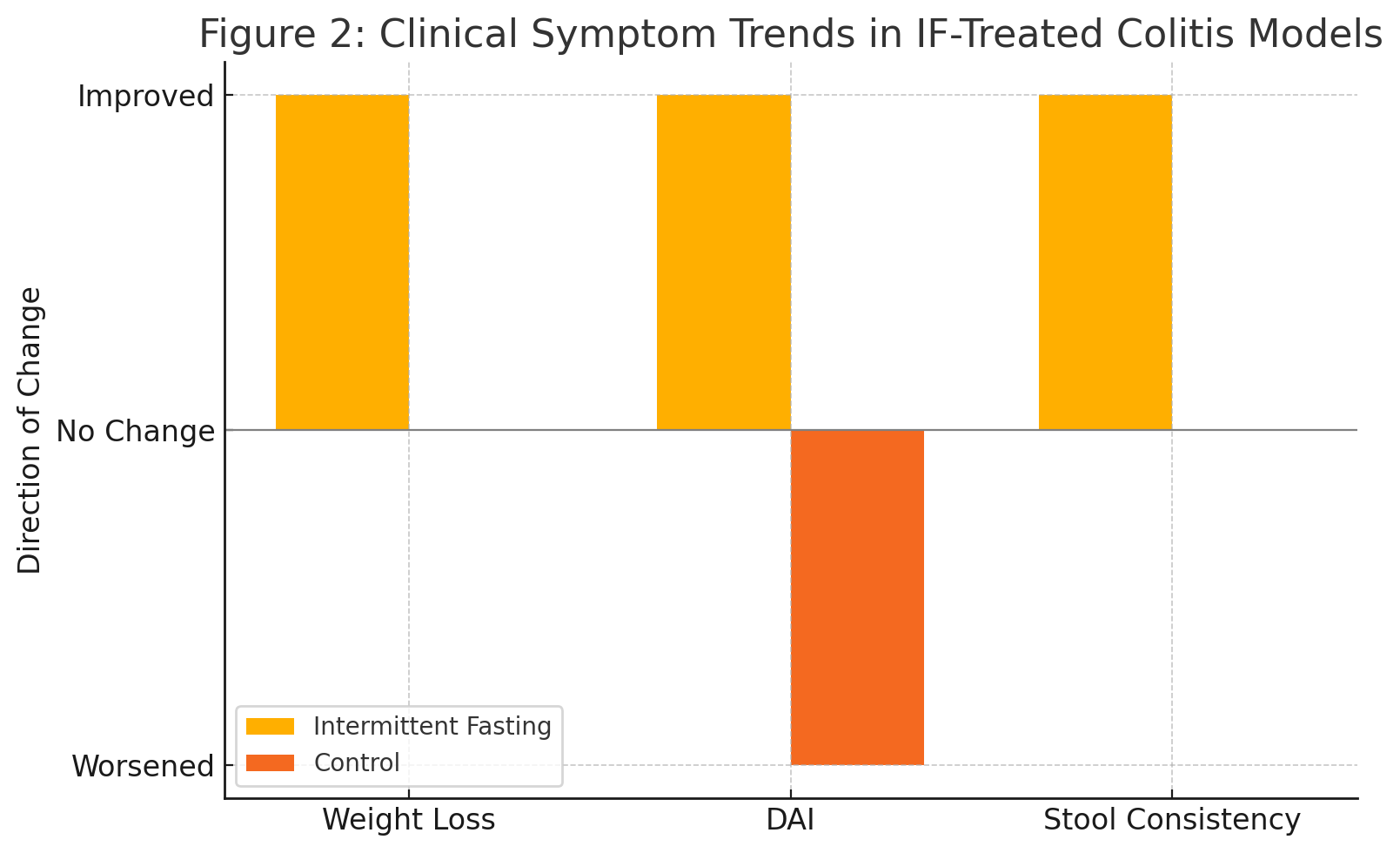
Figure: Figure 2: Clinical Symptom Trends in IF-Treated Colitis Models
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Md. Sanjidul Alam indicated no relevant financial relationships.
Mahanaz Ansa indicated no relevant financial relationships.
Nadia Smita indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Md. Sanjidul Alam, MBBS2, Mahanaz Nabi. Ansa, MBBS3, Nadia Smita, 4, Rishika Trivedi, MD5, Asif Zamir, MD, FACG6. P5355 - Intermittent Fasting Improves Colitis Outcomes in Preclinical IBD Models: A Meta-Analysis to Guide Human Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2Sylhet MAG Osmani Medical College Hospital, Atlantic City, NJ; 3Sylhet MAG Osmani Medical College Hospital, Sylhet, Sylhet, Bangladesh; 4University of Texas Rio Grande Valley, Edinburg, TX; 5DHR Health, McAllen, TX; 6DHR Health Gastroenterology, Edinburg, TX
Introduction: Inflammatory Bowel Disease (IBD), including Crohn’s disease and ulcerative colitis, is a lifelong disorder marked by immune dysregulation, mucosal injury, and microbiome imbalance. While pharmacologic therapies dominate current treatment algorithms, non-drug strategies like intermittent fasting (IF) remain underexplored. This reflects increasing interest in holistic, low-toxicity adjuncts for IBD. Despite emerging animal data, no consolidated evidence exists to guide translational research. This meta-analysis aimed to evaluate the effects of IF on inflammatory, histologic, microbial, and clinical outcomes in preclinical IBD models and assess its readiness for human trial design.
Methods: Following PRISMA guidelines, we conducted a systematic review and meta-analysis across PubMed, Embase, Cochrane Library, ScienceDirect, Google Scholar, and ClinicalTrials.gov (2000–2024) for preclinical or clinical studies evaluating IF in chemically induced colitis. Inclusion criteria required measurement of histologic inflammation, cytokines (e.g., TNF-α, IL-6), microbiota composition, and/or behavioral indicators. Seven murine studies met criteria. Data extraction was independently performed by two reviewers; pooled standardized mean differences (SMD) and qualitative synthesis were used where feasible.
Results:
- Histologic improvement observed in 5/7 studies (pooled SMD: –1.13).
- Pro-inflammatory cytokines (e.g., TNF-α, IL-6) were consistently reduced (SMD range: –1.09 to –1.77).
- Microbial shifts included increased alpha diversity and enrichment of SCFA-producing genera (e.g., Lactobacillus, Bacteroides).
- Behavioral metrics (e.g., weight stabilization, disease activity index) improved across studies.
- No eligible human RCTs were identified, underscoring a critical translational gap.
- Protocol heterogeneity (fasting type, duration) limits uniformity but not reproducibility.
Discussion: Intermittent fasting confers reproducible anti-inflammatory, mucosal, and microbial benefits in preclinical IBD models. The absence of human trials highlights the urgent need for clinical evaluation. These findings offer a mechanistically sound, non-pharmacologic rationale to inform first-in-human studies for IBD remission and adjunctive maintenance.

Figure: Figure 1: Modulation of Inflammatory Cytokines by Intermittent Fasting

Figure: Figure 2: Clinical Symptom Trends in IF-Treated Colitis Models
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Md. Sanjidul Alam indicated no relevant financial relationships.
Mahanaz Ansa indicated no relevant financial relationships.
Nadia Smita indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Md. Sanjidul Alam, MBBS2, Mahanaz Nabi. Ansa, MBBS3, Nadia Smita, 4, Rishika Trivedi, MD5, Asif Zamir, MD, FACG6. P5355 - Intermittent Fasting Improves Colitis Outcomes in Preclinical IBD Models: A Meta-Analysis to Guide Human Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
