Tuesday Poster Session
Category: IBD
P5353 - Neglected Differences: A Meta-Epidemiologic Review of Sex-Disaggregated Outcome Reporting in Ulcerative Colitis Drug Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Nadia Smita, 2, Nabhan Atiquzzaman, 3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5
1DHR Health, Edinburg, Tx, McAllen, TX; 2University of Texas Rio Grande Valley, Edinburg, TX; 3Nova Southeastern University, Fort Lauderdale, FL; 4DHR Health, McAllen, TX; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Sex-based differences shape disease presentation, drug metabolism, and treatment outcomes in ulcerative colitis (UC). While NIH and other regulatory bodies mandate inclusion of sex as a biological variable, clinical trials rarely analyze or report efficacy or safety outcomes by sex. This oversight undermines equitable care and hampers the translation of trial findings into personalized therapy.
To evaluate the frequency and quality of sex-disaggregated outcome reporting in UC drug trials over the past decade and identify factors linked to improved reporting practices.
Methods: We performed a meta-epidemiologic review of UC trials registered on ClinicalTrials.gov from January 1, 2010, to present. Trials were eligible if they included adults (≥18 years), were marked as completed, posted results, and enrolled ≥10 participants. Among 461 inflammatory bowel disease trials screened, 167 UC-specific trials met inclusion criteria. Each was assessed for sex reporting, sex-disaggregated outcome data, and subgroup analysis. Trial characteristics—phase, sponsor type, site geography—were also recorded.
Results: Of 167 UC trials, 92.4% (n=154) reported the sex of enrolled participants. However, only 6 trials (1.4%) provided sex-disaggregated efficacy or safety outcomes. None included pre-specified subgroup analyses by sex. Trials with larger sample sizes, industry sponsorship, and international recruitment were slightly more likely to report sex data, but these traits did not consistently translate into stratified analysis. Discussions of underlying biological or sociocultural mechanisms for sex differences were rare.
Discussion: Despite near-universal reporting of participant sex, UC drug trials overwhelmingly fail to analyze or disclose sex-specific outcomes. This disconnect between policy and practice represents a significant barrier to sex-informed care and precision medicine. Adoption of standardized sex-disaggregated reporting is essential for equitable, evidence-based treatment strategies in UC.
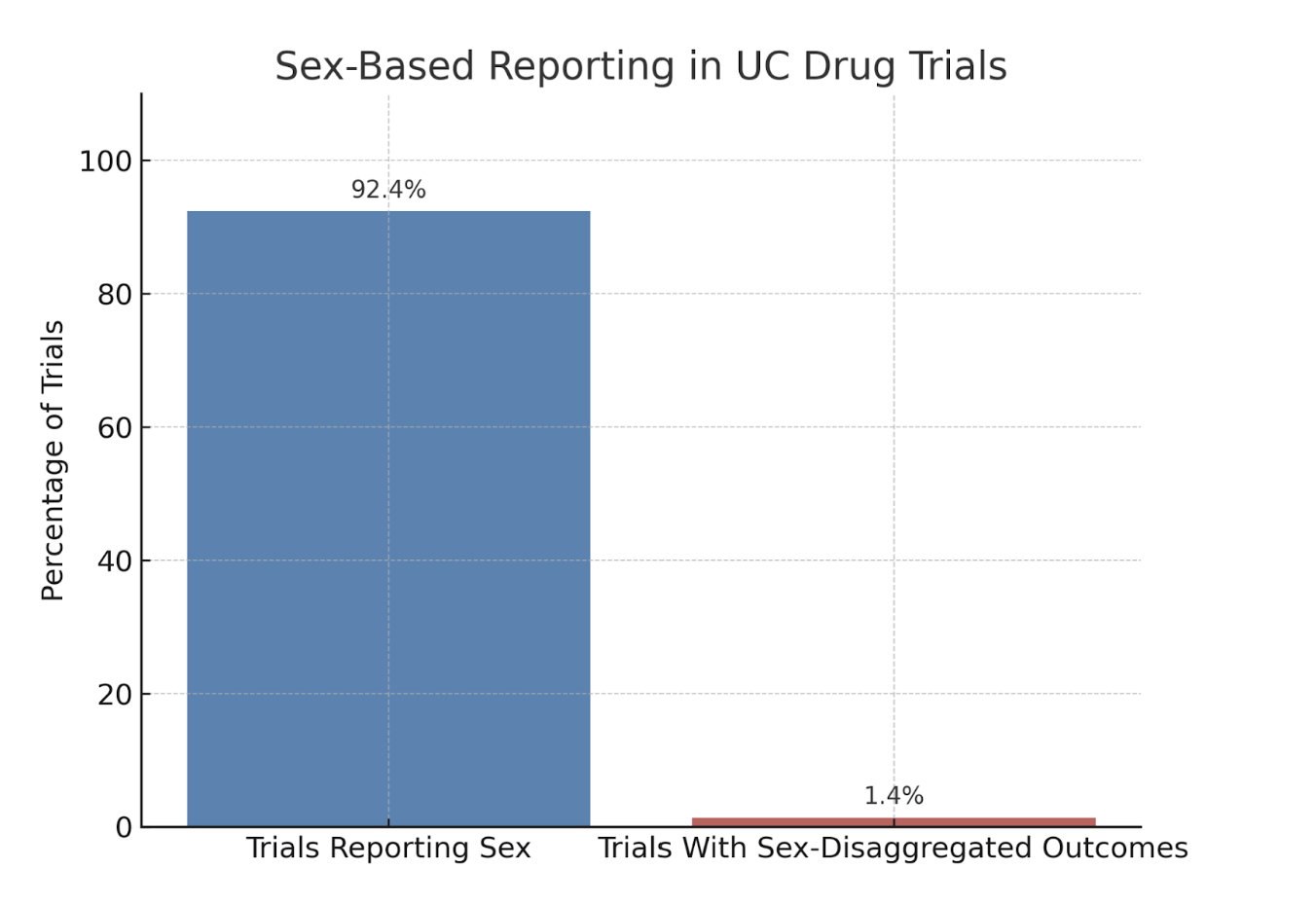
Figure: Figure 1. Proportion of UC drug trials (n=167) that reported participant sex versus those that provided sex-disaggregated efficacy or safety outcomes. While 92.4% of trials documented sex demographics, only 1.4% disaggregated outcome data by sex—highlighting a critical gap in sex-based analysis and reporting.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Nadia Smita indicated no relevant financial relationships.
Nabhan Atiquzzaman indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Nadia Smita, 2, Nabhan Atiquzzaman, 3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5. P5353 - Neglected Differences: A Meta-Epidemiologic Review of Sex-Disaggregated Outcome Reporting in Ulcerative Colitis Drug Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2University of Texas Rio Grande Valley, Edinburg, TX; 3Nova Southeastern University, Fort Lauderdale, FL; 4DHR Health, McAllen, TX; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Sex-based differences shape disease presentation, drug metabolism, and treatment outcomes in ulcerative colitis (UC). While NIH and other regulatory bodies mandate inclusion of sex as a biological variable, clinical trials rarely analyze or report efficacy or safety outcomes by sex. This oversight undermines equitable care and hampers the translation of trial findings into personalized therapy.
To evaluate the frequency and quality of sex-disaggregated outcome reporting in UC drug trials over the past decade and identify factors linked to improved reporting practices.
Methods: We performed a meta-epidemiologic review of UC trials registered on ClinicalTrials.gov from January 1, 2010, to present. Trials were eligible if they included adults (≥18 years), were marked as completed, posted results, and enrolled ≥10 participants. Among 461 inflammatory bowel disease trials screened, 167 UC-specific trials met inclusion criteria. Each was assessed for sex reporting, sex-disaggregated outcome data, and subgroup analysis. Trial characteristics—phase, sponsor type, site geography—were also recorded.
Results: Of 167 UC trials, 92.4% (n=154) reported the sex of enrolled participants. However, only 6 trials (1.4%) provided sex-disaggregated efficacy or safety outcomes. None included pre-specified subgroup analyses by sex. Trials with larger sample sizes, industry sponsorship, and international recruitment were slightly more likely to report sex data, but these traits did not consistently translate into stratified analysis. Discussions of underlying biological or sociocultural mechanisms for sex differences were rare.
Discussion: Despite near-universal reporting of participant sex, UC drug trials overwhelmingly fail to analyze or disclose sex-specific outcomes. This disconnect between policy and practice represents a significant barrier to sex-informed care and precision medicine. Adoption of standardized sex-disaggregated reporting is essential for equitable, evidence-based treatment strategies in UC.

Figure: Figure 1. Proportion of UC drug trials (n=167) that reported participant sex versus those that provided sex-disaggregated efficacy or safety outcomes. While 92.4% of trials documented sex demographics, only 1.4% disaggregated outcome data by sex—highlighting a critical gap in sex-based analysis and reporting.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Nadia Smita indicated no relevant financial relationships.
Nabhan Atiquzzaman indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Nadia Smita, 2, Nabhan Atiquzzaman, 3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5. P5353 - Neglected Differences: A Meta-Epidemiologic Review of Sex-Disaggregated Outcome Reporting in Ulcerative Colitis Drug Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
