Tuesday Poster Session
Category: IBD
P5352 - Where Are the Gaps? A Meta-Epidemiologic Review of Demographic and Global Representation in Ulcerative Colitis Clinical Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Nadia Smita, 2, Nabhan Atiquzzaman, 3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5
1DHR Health, Edinburg, Tx, McAllen, TX; 2University of Texas Rio Grande Valley, Edinburg, TX; 3Nova Southeastern University, Fort Lauderdale, FL; 4DHR Health, McAllen, TX; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Equitable representation in clinical trials is essential for the generalizability of findings and the delivery of precision medicine. Despite mandates from the National Institutes of Health (NIH) and the American College of Gastroenterology (ACG) promoting diversity, the extent to which ulcerative colitis (UC) trials reflect demographic and geographic diversity remains inadequately characterized.
Methods: We conducted a systematic review of ClinicalTrials.gov for trials registered between January 1, 2010, and January 1, 2024, using the terms “ulcerative colitis,” “Crohn’s disease,” and “inflammatory bowel disease.” Trials were included if they enrolled ≥10 adults (≥18 years), were completed with posted results, and focused on UC. From 461 identified studies, 167 UC-specific trials met eligibility criteria. Data were extracted on trial location (US-only, international-only, or both), multi-center status, and reporting of sex, race, and ethnicity. Descriptive statistics and visualization tools (bar charts, forest and funnel plots) were used to assess reporting trends and participant diversity.
Results: Among 167 UC trials, 3.6% were US-only, 6.6% international-only, and 89.8% included both. Most trials (73%) were multi-center. While 94.0% reported participant sex, only 63% reported race or ethnicity. Of those, 35% enrolled cohorts with >80% White participants. Merely 1.4% of studies disaggregated outcomes by sex. Forest plots showed high clustering around White-majority cohorts, and funnel plots revealed low heterogeneity among studies with high precision—highlighting consistent underrepresentation of racial minorities.
Discussion: Despite global reach, UC trials demonstrate substantial gaps in reporting and representation. Underreporting of race/ethnicity, minimal sex-based outcome analysis, and dominance of Western cohorts limit external validity and equity in care delivery. These findings emphasize the urgent need for standardized demographic reporting, inclusion metrics, and diversity-focused design to align with ACG and NIH priorities.
To realize precision medicine in IBD, UC trials must prioritize demographic transparency, inclusive recruitment strategies, and global equity frameworks. Reforming trial design to reflect the diversity of the IBD population is a clinical and ethical imperative.
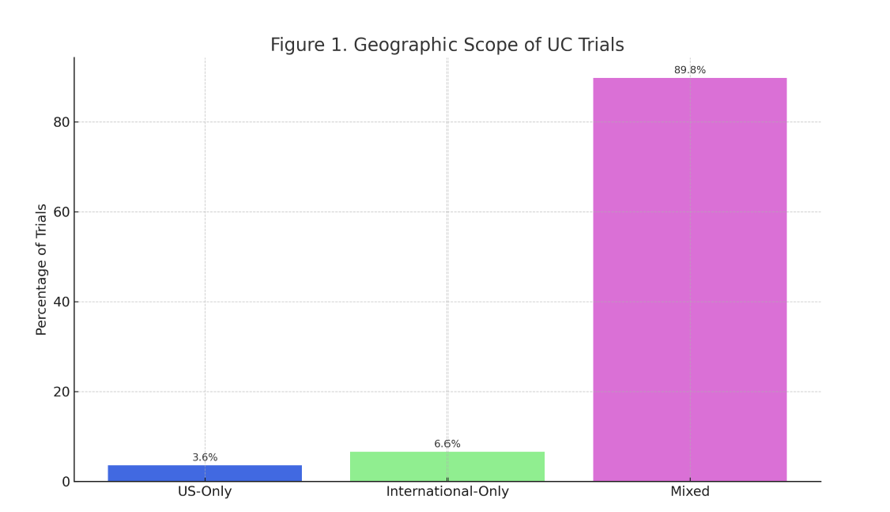
Figure: Figure 1. Geographic Scope of Ulcerative Colitis Trials (2010–2024)
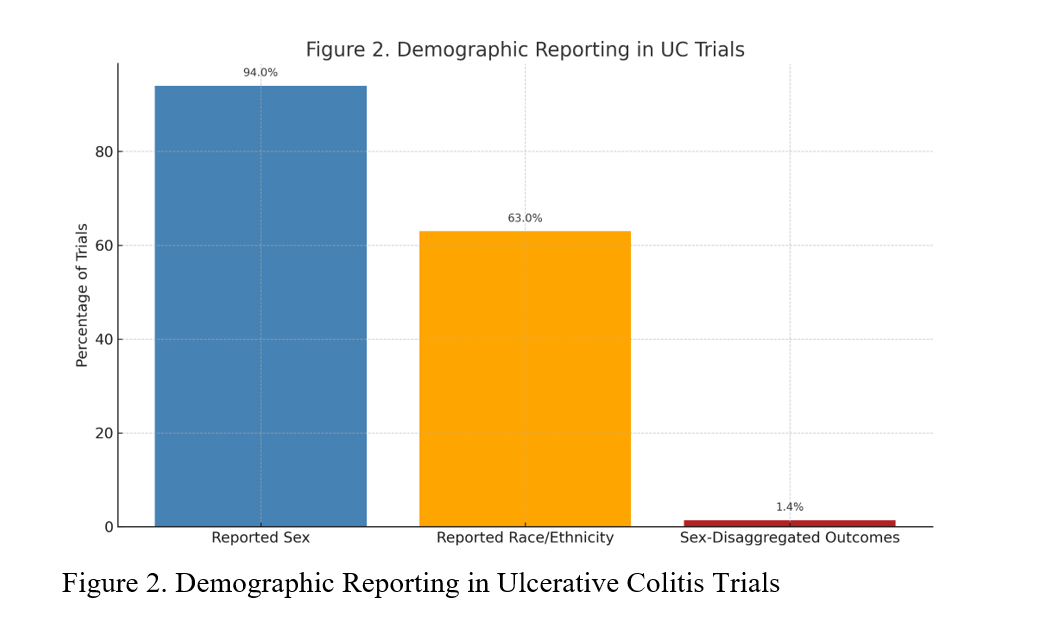
Figure: Figure 2. Demographic Reporting in Ulcerative Colitis Trials
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Nadia Smita indicated no relevant financial relationships.
Nabhan Atiquzzaman indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Nadia Smita, 2, Nabhan Atiquzzaman, 3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5. P5352 - Where Are the Gaps? A Meta-Epidemiologic Review of Demographic and Global Representation in Ulcerative Colitis Clinical Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2University of Texas Rio Grande Valley, Edinburg, TX; 3Nova Southeastern University, Fort Lauderdale, FL; 4DHR Health, McAllen, TX; 5DHR Health Gastroenterology, Edinburg, TX
Introduction: Equitable representation in clinical trials is essential for the generalizability of findings and the delivery of precision medicine. Despite mandates from the National Institutes of Health (NIH) and the American College of Gastroenterology (ACG) promoting diversity, the extent to which ulcerative colitis (UC) trials reflect demographic and geographic diversity remains inadequately characterized.
Methods: We conducted a systematic review of ClinicalTrials.gov for trials registered between January 1, 2010, and January 1, 2024, using the terms “ulcerative colitis,” “Crohn’s disease,” and “inflammatory bowel disease.” Trials were included if they enrolled ≥10 adults (≥18 years), were completed with posted results, and focused on UC. From 461 identified studies, 167 UC-specific trials met eligibility criteria. Data were extracted on trial location (US-only, international-only, or both), multi-center status, and reporting of sex, race, and ethnicity. Descriptive statistics and visualization tools (bar charts, forest and funnel plots) were used to assess reporting trends and participant diversity.
Results: Among 167 UC trials, 3.6% were US-only, 6.6% international-only, and 89.8% included both. Most trials (73%) were multi-center. While 94.0% reported participant sex, only 63% reported race or ethnicity. Of those, 35% enrolled cohorts with >80% White participants. Merely 1.4% of studies disaggregated outcomes by sex. Forest plots showed high clustering around White-majority cohorts, and funnel plots revealed low heterogeneity among studies with high precision—highlighting consistent underrepresentation of racial minorities.
Discussion: Despite global reach, UC trials demonstrate substantial gaps in reporting and representation. Underreporting of race/ethnicity, minimal sex-based outcome analysis, and dominance of Western cohorts limit external validity and equity in care delivery. These findings emphasize the urgent need for standardized demographic reporting, inclusion metrics, and diversity-focused design to align with ACG and NIH priorities.
To realize precision medicine in IBD, UC trials must prioritize demographic transparency, inclusive recruitment strategies, and global equity frameworks. Reforming trial design to reflect the diversity of the IBD population is a clinical and ethical imperative.

Figure: Figure 1. Geographic Scope of Ulcerative Colitis Trials (2010–2024)

Figure: Figure 2. Demographic Reporting in Ulcerative Colitis Trials
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Nadia Smita indicated no relevant financial relationships.
Nabhan Atiquzzaman indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Nadia Smita, 2, Nabhan Atiquzzaman, 3, Rishika Trivedi, MD4, Asif Zamir, MD, FACG5. P5352 - Where Are the Gaps? A Meta-Epidemiologic Review of Demographic and Global Representation in Ulcerative Colitis Clinical Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
