Tuesday Poster Session
Category: IBD
P5325 - Real-World Effectiveness and Safety of Upadacitinib in Ulcerative Colitis: A Multi-Centre Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- AC
Arun Chandnani, MD
Washington University School of Medicine in St. Louis
St. Louis, MO
Presenting Author(s)
Award: ACG Presidential Poster Award
Arun Chandnani, MD1, Katherine Huang, BS1, Abdul Khan, MD2, Sami Samaan, BS1, Prajith Ramesh, MBBS3, Caroline Conlon, MD4, Jeremy A.. Klein, MD5, Nikhil Reddy, MD6, Malek Ayoub, MD1, Gabriela Cervantes, MD2, Melissa Myers, BS2, Martha Bucaram, BS2, Stephen Lieto, MD7, Nicholas Scalzo, MD8, Benjamin L. Cohen, MD9, Matthew Ciorba, MD1, Tingyi Tan, BS1, Richa Shukla, MD10, Anthony Xu, MD10, Navreet Chowla, MD, FACG3, Sina Ogholikhan, MD11, Michelle Kujawski, PhD11, Jae Rok Kim, PharmD, MS12, David Dulaney, MD13, Marc Fenster, MD14, Caroline Benson, BS7, Tamara Alhobayb, MD7, Jeffrey Berinstein, MD, MS15, Shrinivas Bishu, MD15, Anish Patel, MD16, Raina Shivashankar, MD17, Ryan Ungaro, MD, MS18, Amanda Johnson, MD3, Joel Pekow, MD19, Andres Yarur, MD2, Parakkal Deepak, 20
1Washington University School of Medicine in St. Louis, St. Louis, MO; 2Cedars-Sinai Medical Center, Los Angeles, CA; 3Mayo Clinic, Rochester, MN; 4Thomas Jefferson University, Philadelphia, PA; 5University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 6University of Chicago Medicine, Chicago, IL; 7Icahn School of Medicine at Mount Sinai, New York, NY; 8Brown University / Rhode Island Hospital, Providence, RI; 9Cleveland Clinic Foundation, Cleveland, OH; 10Baylor College of Medicine, Houston, TX; 11AbbVie Inc., North Chicago, IL; 12AbbVie Inc, Irvine, CA; 13Brooke Army Medical Center, Fort Sam Houston, TX; 14Montefiore Medical Center, Bronx, NY; 15University of Michigan, Ann Arbor, MI; 16Brooke Army Medical Center, San Antonio, TX; 17Thomas Jefferson University Hospital, Philadelphia, PA; 18Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY; 19University of Chicago, Chicago, IL; 20Department of Medicine, Washington University School of Medicine, St. Louis, MO
Introduction: We aimed to describe the real-world effectiveness and safety of upadacitinib (UPA) in ulcerative colitis (UC).
Methods: We retrospectively analyzed an ongoing multi-center cohort in the United States of UC patients who started on UPA for active disease. Primary outcome was clinical remission at 1-yr assessed by partial Mayo score (resolution of all UC related symptoms and physician global assessment). Secondary outcomes assessed at wk 8 and 6 months (where applicable) included clinical response ( >50% reduction in symptoms utilizing the partial Mayo score), clinical remission, endoscopic response (Mayo endoscopic score ≤1 or absence of erosions/ulcerations) and histological remission (defined as normal or chronic inactive). Descriptive statistics was performed. Adverse events investigated include cardiac events (transient ischemic attack, heart failure, and myocardial infarction), venous thrombus embolism/pulmonary embolism (VTE-PE), and herpes zoster (HZ).
Results: 405 patients (396 UC and 9 IBD-undifferentiated) were included (Table 1) with median age at initiation of 37.00 years (range 27-47) and a median disease duration of 7.00 years (range 3-14). 56% were men, 84.4% white, 9% African American. 64.4% had extensive colitis. 356 (87.9%) had induction for 8 weeks while 17 (4.2) had induction for 16 wks and 393 (93%) had an induction dose of 45 mg. Maintenance dosing was mostly 30 mg (90.6%). Median duration of follow up was 403 days (interquartile range, 206-720) with 97 (23.95%) with follow-up at 1 year or beyond. Among the evaluable, clinical remission at 8 wks was 36.4% (116/319), 6 months was 48.1% (25/52) and 1 year was 80.6 % (29/36). Endoscopic response at 6 months was 80% (16/20) and at 1 year 64% (9/14). Histologic remission was seen at 6 months in 38% (14/36) and at 1 year in 60% (9/15). Colectomy occurred in 19 patients (4.7%). Adverse events were reported in 12.7% with HZ reported in 5 patients (1.5%). One patient aged 53 years had an episode of TIA after 1 year on UPA at 15 mg, without pre-existing cardiovascular risk factors and the UPA use is ongoing at the same dose. There were no reported episodes of worsening of pre-existing cardiovascular disease. There were also no episodes of DVT/PE reported at last follow up.
Discussion: UPA is effective and safe in a real-world clinical setting. Ongoing recruitment will provide further evidence on long term effectiveness and safety.
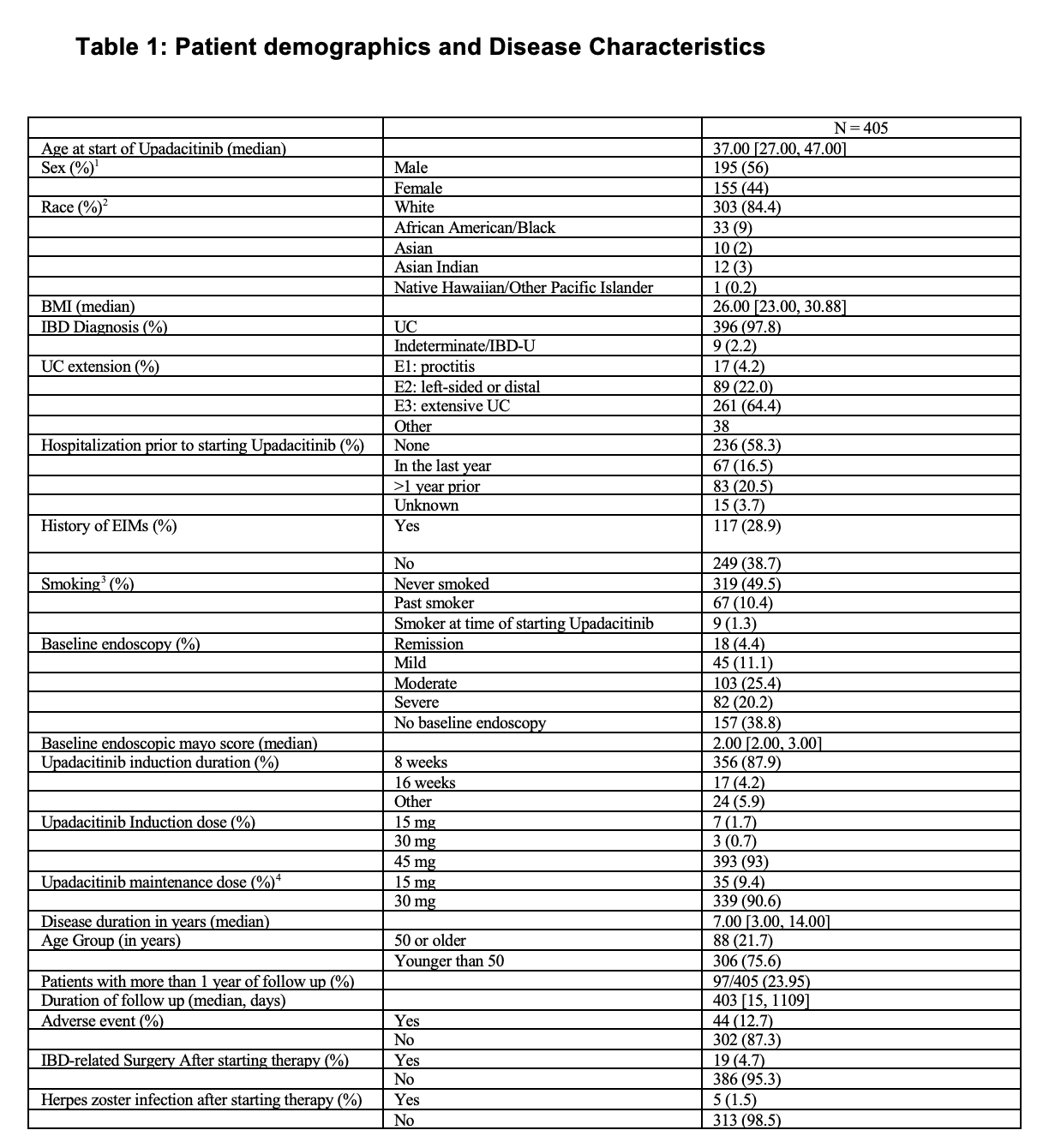
Figure: Table 1. Patient Demographics and Disease Characteristics
Disclosures:
Arun Chandnani indicated no relevant financial relationships.
Katherine Huang indicated no relevant financial relationships.
Abdul Khan indicated no relevant financial relationships.
Sami Samaan indicated no relevant financial relationships.
Prajith Ramesh indicated no relevant financial relationships.
Caroline Conlon indicated no relevant financial relationships.
Jeremy Klein indicated no relevant financial relationships.
Nikhil Reddy indicated no relevant financial relationships.
Malek Ayoub indicated no relevant financial relationships.
Gabriela Cervantes indicated no relevant financial relationships.
Melissa Myers indicated no relevant financial relationships.
Martha Bucaram indicated no relevant financial relationships.
Stephen Lieto indicated no relevant financial relationships.
Nicholas Scalzo indicated no relevant financial relationships.
Benjamin Cohen: Abbvie – Advisory Committee/Board Member, Consultant, Speakers Bureau. ALPCO – Advisory Committee/Board Member, Consultant. Emmes Biopharma Services LLC – DSMB. J&J Innovative Medicine – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Consultant, Speakers Bureau.
Matthew Ciorba indicated no relevant financial relationships.
Tingyi Tan indicated no relevant financial relationships.
Richa Shukla: Abbvie, Lilly – Speakers Bureau.
Anthony Xu indicated no relevant financial relationships.
Navreet Chowla indicated no relevant financial relationships.
Sina Ogholikhan: AbbVie – Employee, Stock Options.
Michelle Kujawski: AbbVie – Employee, Stock Options.
Jae Rok Kim: AbbVie – Employee, Stock Options.
David Dulaney indicated no relevant financial relationships.
Marc Fenster indicated no relevant financial relationships.
Caroline Benson indicated no relevant financial relationships.
Tamara Alhobayb indicated no relevant financial relationships.
Jeffrey Berinstein indicated no relevant financial relationships.
Shrinivas Bishu indicated no relevant financial relationships.
Anish Patel indicated no relevant financial relationships.
Raina Shivashankar: Abbvie – Speakers Bureau. BMS – Speakers Bureau. Janssen – Grant/Research Support. Pfizer – Consultant.
Ryan Ungaro: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Lilly – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant.
Amanda Johnson indicated no relevant financial relationships.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
Andres Yarur indicated no relevant financial relationships.
Parakkal Deepak: Arena Pharmaceuticals – Grant/Research Support, Personal or other fees. Boehringer Ingelheim – Grant/Research Support, Personal or other fees. Bristol Myers Squibb/Celgene – Grant/Research Support, Personal or other fees. Janssen – Grant/Research Support, Personal or other fees. Pfizer – Grant/Research Support, Personal or other fees. Prometheus Biosciences – Grant/Research Support, Personal or other fees. Takeda – Grant/Research Support, Personal or other fees.
Arun Chandnani, MD1, Katherine Huang, BS1, Abdul Khan, MD2, Sami Samaan, BS1, Prajith Ramesh, MBBS3, Caroline Conlon, MD4, Jeremy A.. Klein, MD5, Nikhil Reddy, MD6, Malek Ayoub, MD1, Gabriela Cervantes, MD2, Melissa Myers, BS2, Martha Bucaram, BS2, Stephen Lieto, MD7, Nicholas Scalzo, MD8, Benjamin L. Cohen, MD9, Matthew Ciorba, MD1, Tingyi Tan, BS1, Richa Shukla, MD10, Anthony Xu, MD10, Navreet Chowla, MD, FACG3, Sina Ogholikhan, MD11, Michelle Kujawski, PhD11, Jae Rok Kim, PharmD, MS12, David Dulaney, MD13, Marc Fenster, MD14, Caroline Benson, BS7, Tamara Alhobayb, MD7, Jeffrey Berinstein, MD, MS15, Shrinivas Bishu, MD15, Anish Patel, MD16, Raina Shivashankar, MD17, Ryan Ungaro, MD, MS18, Amanda Johnson, MD3, Joel Pekow, MD19, Andres Yarur, MD2, Parakkal Deepak, 20. P5325 - Real-World Effectiveness and Safety of Upadacitinib in Ulcerative Colitis: A Multi-Centre Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Arun Chandnani, MD1, Katherine Huang, BS1, Abdul Khan, MD2, Sami Samaan, BS1, Prajith Ramesh, MBBS3, Caroline Conlon, MD4, Jeremy A.. Klein, MD5, Nikhil Reddy, MD6, Malek Ayoub, MD1, Gabriela Cervantes, MD2, Melissa Myers, BS2, Martha Bucaram, BS2, Stephen Lieto, MD7, Nicholas Scalzo, MD8, Benjamin L. Cohen, MD9, Matthew Ciorba, MD1, Tingyi Tan, BS1, Richa Shukla, MD10, Anthony Xu, MD10, Navreet Chowla, MD, FACG3, Sina Ogholikhan, MD11, Michelle Kujawski, PhD11, Jae Rok Kim, PharmD, MS12, David Dulaney, MD13, Marc Fenster, MD14, Caroline Benson, BS7, Tamara Alhobayb, MD7, Jeffrey Berinstein, MD, MS15, Shrinivas Bishu, MD15, Anish Patel, MD16, Raina Shivashankar, MD17, Ryan Ungaro, MD, MS18, Amanda Johnson, MD3, Joel Pekow, MD19, Andres Yarur, MD2, Parakkal Deepak, 20
1Washington University School of Medicine in St. Louis, St. Louis, MO; 2Cedars-Sinai Medical Center, Los Angeles, CA; 3Mayo Clinic, Rochester, MN; 4Thomas Jefferson University, Philadelphia, PA; 5University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 6University of Chicago Medicine, Chicago, IL; 7Icahn School of Medicine at Mount Sinai, New York, NY; 8Brown University / Rhode Island Hospital, Providence, RI; 9Cleveland Clinic Foundation, Cleveland, OH; 10Baylor College of Medicine, Houston, TX; 11AbbVie Inc., North Chicago, IL; 12AbbVie Inc, Irvine, CA; 13Brooke Army Medical Center, Fort Sam Houston, TX; 14Montefiore Medical Center, Bronx, NY; 15University of Michigan, Ann Arbor, MI; 16Brooke Army Medical Center, San Antonio, TX; 17Thomas Jefferson University Hospital, Philadelphia, PA; 18Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY; 19University of Chicago, Chicago, IL; 20Department of Medicine, Washington University School of Medicine, St. Louis, MO
Introduction: We aimed to describe the real-world effectiveness and safety of upadacitinib (UPA) in ulcerative colitis (UC).
Methods: We retrospectively analyzed an ongoing multi-center cohort in the United States of UC patients who started on UPA for active disease. Primary outcome was clinical remission at 1-yr assessed by partial Mayo score (resolution of all UC related symptoms and physician global assessment). Secondary outcomes assessed at wk 8 and 6 months (where applicable) included clinical response ( >50% reduction in symptoms utilizing the partial Mayo score), clinical remission, endoscopic response (Mayo endoscopic score ≤1 or absence of erosions/ulcerations) and histological remission (defined as normal or chronic inactive). Descriptive statistics was performed. Adverse events investigated include cardiac events (transient ischemic attack, heart failure, and myocardial infarction), venous thrombus embolism/pulmonary embolism (VTE-PE), and herpes zoster (HZ).
Results: 405 patients (396 UC and 9 IBD-undifferentiated) were included (Table 1) with median age at initiation of 37.00 years (range 27-47) and a median disease duration of 7.00 years (range 3-14). 56% were men, 84.4% white, 9% African American. 64.4% had extensive colitis. 356 (87.9%) had induction for 8 weeks while 17 (4.2) had induction for 16 wks and 393 (93%) had an induction dose of 45 mg. Maintenance dosing was mostly 30 mg (90.6%). Median duration of follow up was 403 days (interquartile range, 206-720) with 97 (23.95%) with follow-up at 1 year or beyond. Among the evaluable, clinical remission at 8 wks was 36.4% (116/319), 6 months was 48.1% (25/52) and 1 year was 80.6 % (29/36). Endoscopic response at 6 months was 80% (16/20) and at 1 year 64% (9/14). Histologic remission was seen at 6 months in 38% (14/36) and at 1 year in 60% (9/15). Colectomy occurred in 19 patients (4.7%). Adverse events were reported in 12.7% with HZ reported in 5 patients (1.5%). One patient aged 53 years had an episode of TIA after 1 year on UPA at 15 mg, without pre-existing cardiovascular risk factors and the UPA use is ongoing at the same dose. There were no reported episodes of worsening of pre-existing cardiovascular disease. There were also no episodes of DVT/PE reported at last follow up.
Discussion: UPA is effective and safe in a real-world clinical setting. Ongoing recruitment will provide further evidence on long term effectiveness and safety.

Figure: Table 1. Patient Demographics and Disease Characteristics
Disclosures:
Arun Chandnani indicated no relevant financial relationships.
Katherine Huang indicated no relevant financial relationships.
Abdul Khan indicated no relevant financial relationships.
Sami Samaan indicated no relevant financial relationships.
Prajith Ramesh indicated no relevant financial relationships.
Caroline Conlon indicated no relevant financial relationships.
Jeremy Klein indicated no relevant financial relationships.
Nikhil Reddy indicated no relevant financial relationships.
Malek Ayoub indicated no relevant financial relationships.
Gabriela Cervantes indicated no relevant financial relationships.
Melissa Myers indicated no relevant financial relationships.
Martha Bucaram indicated no relevant financial relationships.
Stephen Lieto indicated no relevant financial relationships.
Nicholas Scalzo indicated no relevant financial relationships.
Benjamin Cohen: Abbvie – Advisory Committee/Board Member, Consultant, Speakers Bureau. ALPCO – Advisory Committee/Board Member, Consultant. Emmes Biopharma Services LLC – DSMB. J&J Innovative Medicine – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Consultant, Speakers Bureau.
Matthew Ciorba indicated no relevant financial relationships.
Tingyi Tan indicated no relevant financial relationships.
Richa Shukla: Abbvie, Lilly – Speakers Bureau.
Anthony Xu indicated no relevant financial relationships.
Navreet Chowla indicated no relevant financial relationships.
Sina Ogholikhan: AbbVie – Employee, Stock Options.
Michelle Kujawski: AbbVie – Employee, Stock Options.
Jae Rok Kim: AbbVie – Employee, Stock Options.
David Dulaney indicated no relevant financial relationships.
Marc Fenster indicated no relevant financial relationships.
Caroline Benson indicated no relevant financial relationships.
Tamara Alhobayb indicated no relevant financial relationships.
Jeffrey Berinstein indicated no relevant financial relationships.
Shrinivas Bishu indicated no relevant financial relationships.
Anish Patel indicated no relevant financial relationships.
Raina Shivashankar: Abbvie – Speakers Bureau. BMS – Speakers Bureau. Janssen – Grant/Research Support. Pfizer – Consultant.
Ryan Ungaro: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Lilly – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant.
Amanda Johnson indicated no relevant financial relationships.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
Andres Yarur indicated no relevant financial relationships.
Parakkal Deepak: Arena Pharmaceuticals – Grant/Research Support, Personal or other fees. Boehringer Ingelheim – Grant/Research Support, Personal or other fees. Bristol Myers Squibb/Celgene – Grant/Research Support, Personal or other fees. Janssen – Grant/Research Support, Personal or other fees. Pfizer – Grant/Research Support, Personal or other fees. Prometheus Biosciences – Grant/Research Support, Personal or other fees. Takeda – Grant/Research Support, Personal or other fees.
Arun Chandnani, MD1, Katherine Huang, BS1, Abdul Khan, MD2, Sami Samaan, BS1, Prajith Ramesh, MBBS3, Caroline Conlon, MD4, Jeremy A.. Klein, MD5, Nikhil Reddy, MD6, Malek Ayoub, MD1, Gabriela Cervantes, MD2, Melissa Myers, BS2, Martha Bucaram, BS2, Stephen Lieto, MD7, Nicholas Scalzo, MD8, Benjamin L. Cohen, MD9, Matthew Ciorba, MD1, Tingyi Tan, BS1, Richa Shukla, MD10, Anthony Xu, MD10, Navreet Chowla, MD, FACG3, Sina Ogholikhan, MD11, Michelle Kujawski, PhD11, Jae Rok Kim, PharmD, MS12, David Dulaney, MD13, Marc Fenster, MD14, Caroline Benson, BS7, Tamara Alhobayb, MD7, Jeffrey Berinstein, MD, MS15, Shrinivas Bishu, MD15, Anish Patel, MD16, Raina Shivashankar, MD17, Ryan Ungaro, MD, MS18, Amanda Johnson, MD3, Joel Pekow, MD19, Andres Yarur, MD2, Parakkal Deepak, 20. P5325 - Real-World Effectiveness and Safety of Upadacitinib in Ulcerative Colitis: A Multi-Centre Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

