Tuesday Poster Session
Category: IBD
P5308 - Safety and Efficacy of Guselkumab in the Treatment of Inflammatory Bowel Diseases: A Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Marina Takawy, MD
Rochester Regional Health, Unity Hospital
Rochester, NY
Presenting Author(s)
Salma Allam, 1, Mohamed Saad Sayed, MBBCh2, Bishoy Fahim, MBBCh3, Ibrahim Mohammed, MD4, Marina Takawy, MD5, Mohammed Al-Aquily, MD6, Abdallfatah Abdallfatah, 7, Fatma El Badrawy, 8, Nermin Elhossiny, 9, Mohamed A. Aldemerdash, 10, Dalia Atef. Abouda, 11, Hazem Abosheaishaa, MD12
1Galala University, Suez, As Suways, Egypt; 2Faculty of Medicine, Ihnasya, Bani Suwayf, Egypt; 3Sohag University Hospital, Sohag, Suhaj, Egypt; 4Albany Medical Center, Albany, NY; 5Rochester Regional Health, Unity Hospital, Rochester, NY; 6Norwalk Hospital/Yale University, Norwalk, CT; 7Faculty of Medicine, October 6 University, Giza, Al Jizah, Egypt; 8Misr University for Science & Technology, Sheikh Zayed, Al Jizah, Egypt; 9St Mary General hospital prime healthcare , NJ , USA, Passaic, NJ; 10Sohag University, Faculty of Medicine, Sohag, Suhaj, Egypt; 11Alexandria University, Beheria, Abu Matamir, Al Buhayrah, Egypt; 12Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY
Introduction: Guselkumab, a human monoclonal antibody targeting the interleukin-23 p19 subunit, has emerged as a promising therapeutic option for inflammatory bowel disease (IBD). By inhibiting IL-23, Guselkumab modulates key inflammatory pathways implicated in IBD pathogenesis. While recent randomized controlled trials (RCTs) have reported its efficacy and favorable safety profile, the collective evidence remains to be comprehensively evaluated. This meta-analysis aimed to assess the safety and efficacy of Guselkumab as induction and maintenance therapy in patients with IBD.
Methods: We systematically searched databases including Embase, Scopus, Web of Science, Medline, and Cochrane on 6th April 2025 without any automated filters or language restrictions. RCTs that compared guselkumab with placebo in IBD patients were eligible for inclusion. Pooling of the studies was performed by using the Risk Ratio (RR) with the corresponding 95% Confidence Interval (CI) using a random effects model. All analyses were done by STATA Corp M17.
Results: A total of 1916 patients with a mean age ranged from 35.1 to 43.3 years were pooled through five RCTs. Use of the guselkumab as an induction therapy achieved higher clinical remission [RR: 2.32, 95% CI [1.89; 2.85], p< 0.001, I2 = 0%], and clinical response [RR: 1.81, 95% CI [1.59; 2.07], p< 0.001, I2 = 0%] at 12 weeks. Also, as maintenance therapy, it achieved higher clinical remission [RR: 2.19, 95% CI [1.76; 2.74], p< 0.001, I2 = 0%], and clinical response [RR: 1.62, 95% CI [1.34; 1.95], p< 0.001, I2 = 0%] in 12 weeks. The safety outcomes, including rates of serious adverse events and infections, were comparable between guselkumab and placebo.
Discussion: This meta-analysis underscores the higher efficacy of guselkumab in treating IBD patients within a good safety profile. Future research is needed to clarify the long-term effects of guselkumab regarding its safety.
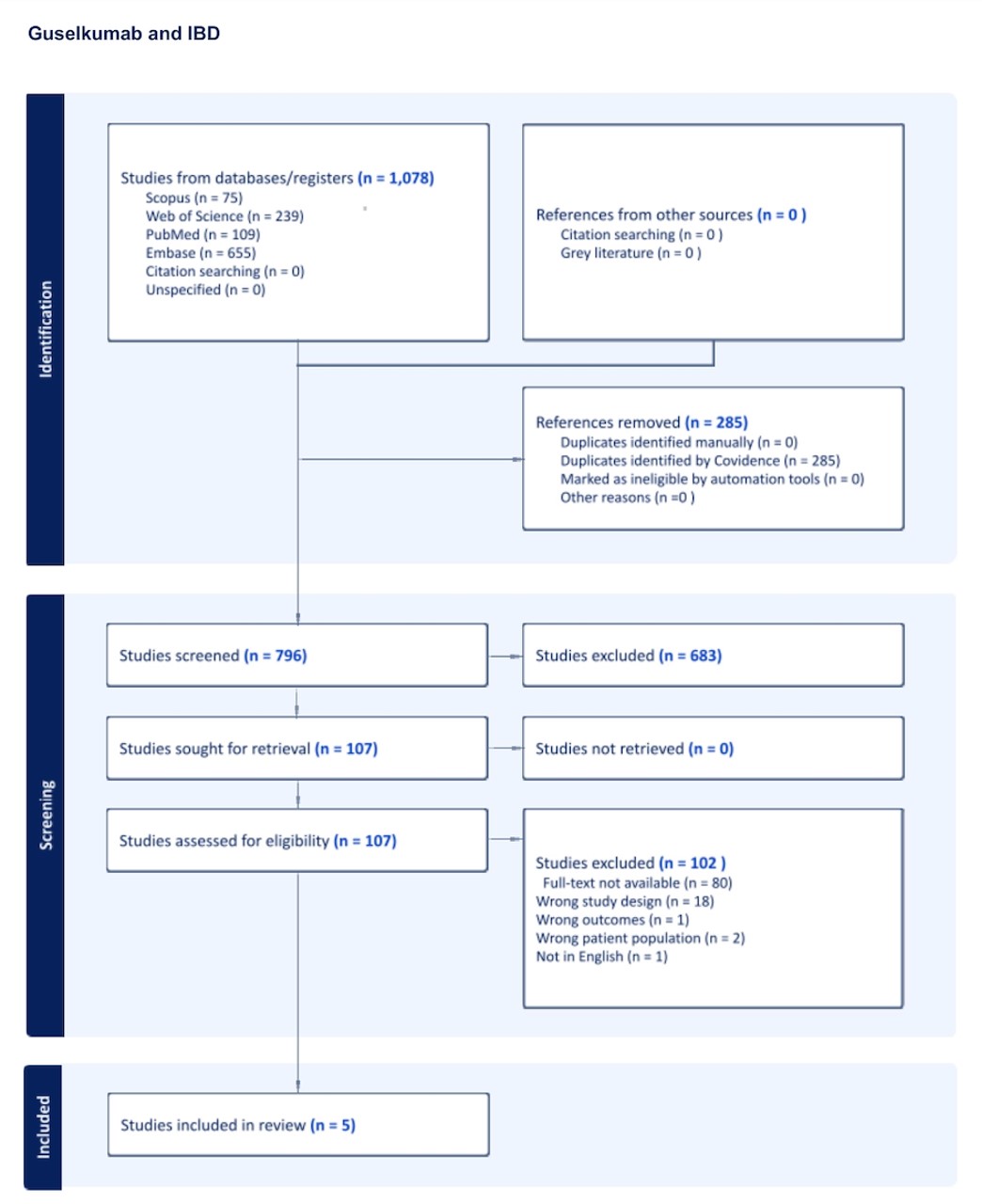
Figure: Figure 1. PRISMA flow chart showing different stages of screening and number of studies included.
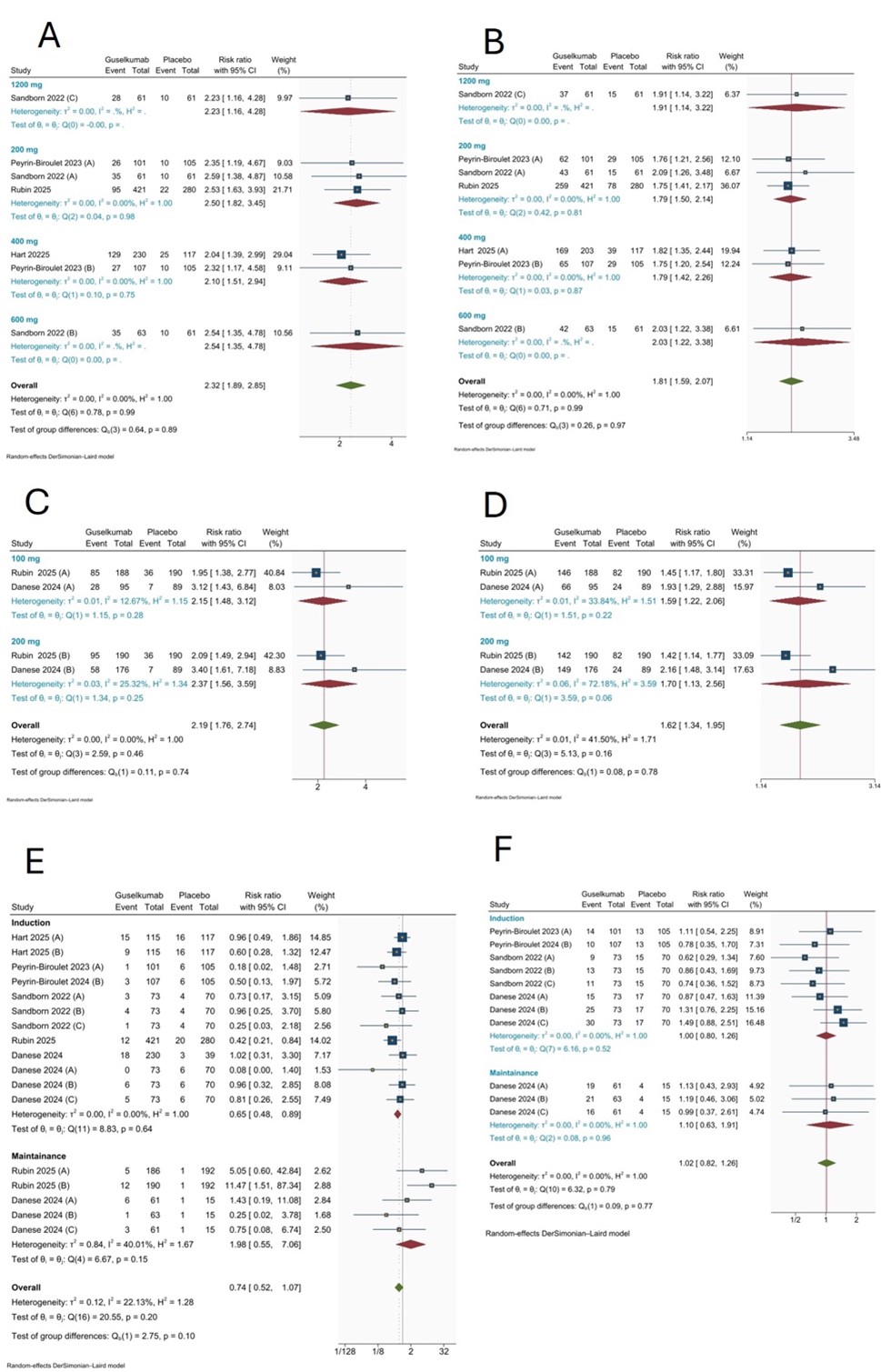
Figure: Figure 2: Meta-analysis Forest plot for A) Clinical remission after 12 weeks induction dose of guselkumab; B) Clinical response after 12 weeks induction dose of guselkumab; C) Clinical remission after 12 weeks maintenance dose of guselkumab; D) Clinical remission after 12 weeks maintenance dose of guselkumab; E) Serious adverse effects after the guselkumab administration; F) Infection after the guselkumab administration (Random-effects model).
Disclosures:
Salma Allam indicated no relevant financial relationships.
Mohamed Saad Sayed indicated no relevant financial relationships.
Bishoy Fahim indicated no relevant financial relationships.
Ibrahim Mohammed indicated no relevant financial relationships.
Marina Takawy indicated no relevant financial relationships.
Mohammed Al-Aquily indicated no relevant financial relationships.
Abdallfatah Abdallfatah indicated no relevant financial relationships.
Fatma El Badrawy indicated no relevant financial relationships.
Nermin Elhossiny indicated no relevant financial relationships.
Mohamed A. Aldemerdash indicated no relevant financial relationships.
Dalia Abouda indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Salma Allam, 1, Mohamed Saad Sayed, MBBCh2, Bishoy Fahim, MBBCh3, Ibrahim Mohammed, MD4, Marina Takawy, MD5, Mohammed Al-Aquily, MD6, Abdallfatah Abdallfatah, 7, Fatma El Badrawy, 8, Nermin Elhossiny, 9, Mohamed A. Aldemerdash, 10, Dalia Atef. Abouda, 11, Hazem Abosheaishaa, MD12. P5308 - Safety and Efficacy of Guselkumab in the Treatment of Inflammatory Bowel Diseases: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Galala University, Suez, As Suways, Egypt; 2Faculty of Medicine, Ihnasya, Bani Suwayf, Egypt; 3Sohag University Hospital, Sohag, Suhaj, Egypt; 4Albany Medical Center, Albany, NY; 5Rochester Regional Health, Unity Hospital, Rochester, NY; 6Norwalk Hospital/Yale University, Norwalk, CT; 7Faculty of Medicine, October 6 University, Giza, Al Jizah, Egypt; 8Misr University for Science & Technology, Sheikh Zayed, Al Jizah, Egypt; 9St Mary General hospital prime healthcare , NJ , USA, Passaic, NJ; 10Sohag University, Faculty of Medicine, Sohag, Suhaj, Egypt; 11Alexandria University, Beheria, Abu Matamir, Al Buhayrah, Egypt; 12Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY
Introduction: Guselkumab, a human monoclonal antibody targeting the interleukin-23 p19 subunit, has emerged as a promising therapeutic option for inflammatory bowel disease (IBD). By inhibiting IL-23, Guselkumab modulates key inflammatory pathways implicated in IBD pathogenesis. While recent randomized controlled trials (RCTs) have reported its efficacy and favorable safety profile, the collective evidence remains to be comprehensively evaluated. This meta-analysis aimed to assess the safety and efficacy of Guselkumab as induction and maintenance therapy in patients with IBD.
Methods: We systematically searched databases including Embase, Scopus, Web of Science, Medline, and Cochrane on 6th April 2025 without any automated filters or language restrictions. RCTs that compared guselkumab with placebo in IBD patients were eligible for inclusion. Pooling of the studies was performed by using the Risk Ratio (RR) with the corresponding 95% Confidence Interval (CI) using a random effects model. All analyses were done by STATA Corp M17.
Results: A total of 1916 patients with a mean age ranged from 35.1 to 43.3 years were pooled through five RCTs. Use of the guselkumab as an induction therapy achieved higher clinical remission [RR: 2.32, 95% CI [1.89; 2.85], p< 0.001, I2 = 0%], and clinical response [RR: 1.81, 95% CI [1.59; 2.07], p< 0.001, I2 = 0%] at 12 weeks. Also, as maintenance therapy, it achieved higher clinical remission [RR: 2.19, 95% CI [1.76; 2.74], p< 0.001, I2 = 0%], and clinical response [RR: 1.62, 95% CI [1.34; 1.95], p< 0.001, I2 = 0%] in 12 weeks. The safety outcomes, including rates of serious adverse events and infections, were comparable between guselkumab and placebo.
Discussion: This meta-analysis underscores the higher efficacy of guselkumab in treating IBD patients within a good safety profile. Future research is needed to clarify the long-term effects of guselkumab regarding its safety.

Figure: Figure 1. PRISMA flow chart showing different stages of screening and number of studies included.

Figure: Figure 2: Meta-analysis Forest plot for A) Clinical remission after 12 weeks induction dose of guselkumab; B) Clinical response after 12 weeks induction dose of guselkumab; C) Clinical remission after 12 weeks maintenance dose of guselkumab; D) Clinical remission after 12 weeks maintenance dose of guselkumab; E) Serious adverse effects after the guselkumab administration; F) Infection after the guselkumab administration (Random-effects model).
Disclosures:
Salma Allam indicated no relevant financial relationships.
Mohamed Saad Sayed indicated no relevant financial relationships.
Bishoy Fahim indicated no relevant financial relationships.
Ibrahim Mohammed indicated no relevant financial relationships.
Marina Takawy indicated no relevant financial relationships.
Mohammed Al-Aquily indicated no relevant financial relationships.
Abdallfatah Abdallfatah indicated no relevant financial relationships.
Fatma El Badrawy indicated no relevant financial relationships.
Nermin Elhossiny indicated no relevant financial relationships.
Mohamed A. Aldemerdash indicated no relevant financial relationships.
Dalia Abouda indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Salma Allam, 1, Mohamed Saad Sayed, MBBCh2, Bishoy Fahim, MBBCh3, Ibrahim Mohammed, MD4, Marina Takawy, MD5, Mohammed Al-Aquily, MD6, Abdallfatah Abdallfatah, 7, Fatma El Badrawy, 8, Nermin Elhossiny, 9, Mohamed A. Aldemerdash, 10, Dalia Atef. Abouda, 11, Hazem Abosheaishaa, MD12. P5308 - Safety and Efficacy of Guselkumab in the Treatment of Inflammatory Bowel Diseases: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

