Tuesday Poster Session
Category: GI Bleeding
P5215 - From Bleeding to Healing: Unveiling the Efficacy and Safety of UI-EWD Hemostatic Powder in Gastrointestinal Bleeding - A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Khadija Mohib, MD (she/her/hers)
Kirk Kerkorian School of Medicine at the University of Nevada Las Vegas
Las Vegas, NV
Presenting Author(s)
Abu-Bakr Ahmed, BA1, Khadija Mohib, MD1, Zain Ul Abideen, MBBS2, Muhammad Hassan Waseem, MBBS3, Sania Aimen, MBBS4, Noor Ul Huda Ramzan, MD5, Mian Uman Anwer, MBBS6, Prasun K.. Jalal, MD7
1Kirk Kerkorian School of Medicine at the University of Nevada Las Vegas, Las Vegas, NV; 2King Edward Medical University, Lahore, Punjab, Pakistan; 3Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 4Quetta Institute of Medical Sciences, Quetta, Balochistan, Pakistan; 5University of Texas Southwestern Medical Center, Dallas, TX; 6Punjab Medical College, Faisalabad, Punjab, Pakistan; 7Baylor College of Medicine, Houston, TX
Introduction: A novel endoscopic hemostatic adhesive powder (UI-EWD) was developed to cope with the high rebleeding rates associated with the current endoscopic hemostatic options for gastrointestinal bleeding. This meta-analysis aimed to assess the safety and effectiveness of UI-EWD hemostatic adhesive powder as a salvage treatment modality for upper and lower gastrointestinal bleeding.
Methods: Electronic databases like PubMed, Cochrane Central and ScienceDirect were searched from inception to January 2025. This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The proportions were pooled with 95 % Confidence Intervals (CI) using R version 4.2.3 and employing the “metaprop” package for the dichotomous outcomes. The primary and secondary outcomes of interest were clinical success, overall rebleeding, early rebleeding, delayed rebleeding, time to rebleeding, mortality and adverse events. The quality assessment was done through the Cochrane Risk of Bias (RoB) 2.0 tool and the Newcastle Ottawa Scale. The risk of publication bias was assessed visually through funnel plots and statistically through Egger’s regression test.
Results: Three studies (including two trials and one cohort) with a total of 211 patients were included in this meta-analysis. The pooled clinical success rate was 98% (95% confidence interval:[85-100 %]; I2 = 0%). The overall rebleeding rate was 11% (95% confidence interval:[0-93%]; I2=11.5% ). The pooled rates of early and delayed rebleeding were 10% (95%CI:[1- 51 %]; I2=70.5%) and 9% (95%CI:[3 - 22%]; I2=0%) respectively.
Discussion: UI-EWD hemostatic adhesive powder demonstrates a high clinical success rate and relatively low overall rebleeding rates, making it an effective salvage treatment for gastrointestinal bleeding. The early and delayed rebleeding rates are manageable, with minimal heterogeneity observed in delayed rebleeding outcomes. These findings suggest UI-EWD is a promising and safe option for managing challenging bleeding cases
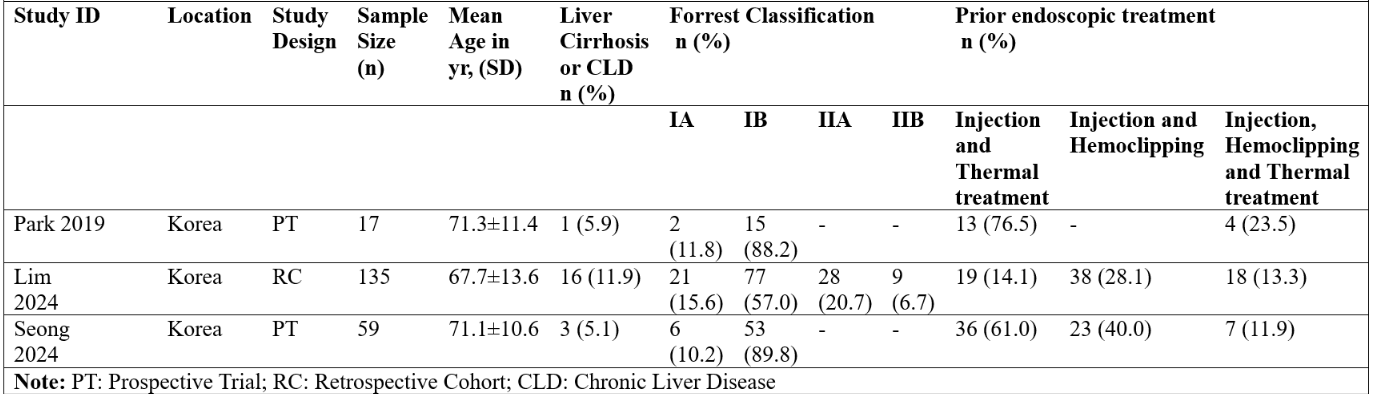
Figure: Figure 1: Forest Plots for (A)Clinical Success (B)Early Rebleeding (C)Delayed Rebleeding (D)Overall Rebleeding
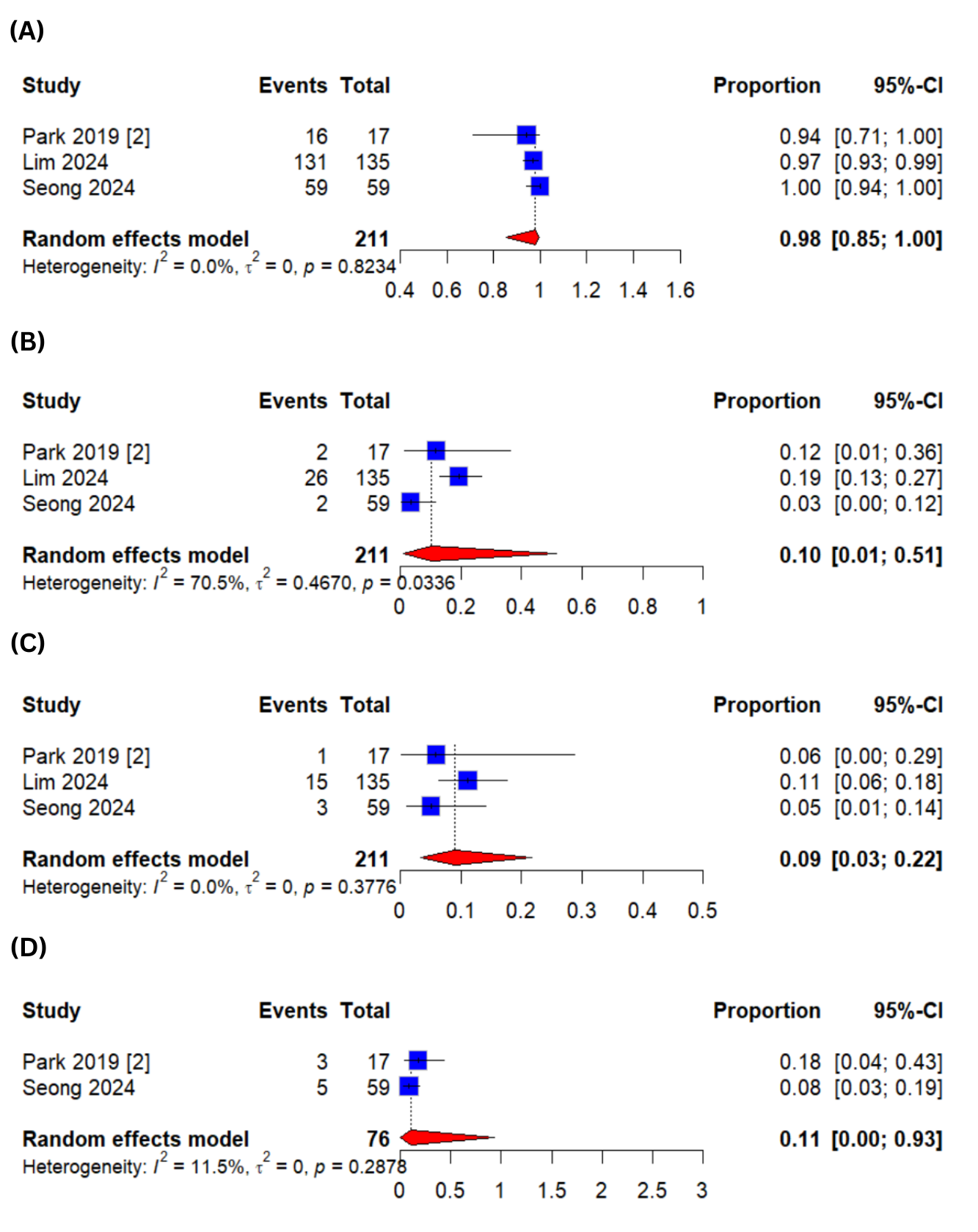
Figure: Figure 2: Baseline Characteristics of the Included Studies
Disclosures:
Abu-Bakr Ahmed indicated no relevant financial relationships.
Khadija Mohib indicated no relevant financial relationships.
Zain Ul Abideen indicated no relevant financial relationships.
Muhammad Hassan Waseem indicated no relevant financial relationships.
Sania Aimen indicated no relevant financial relationships.
Noor Ul Huda Ramzan indicated no relevant financial relationships.
Mian Uman Anwer indicated no relevant financial relationships.
Prasun Jalal: AbbVie – Consultant. Gilead Sciences – Consultant.
Abu-Bakr Ahmed, BA1, Khadija Mohib, MD1, Zain Ul Abideen, MBBS2, Muhammad Hassan Waseem, MBBS3, Sania Aimen, MBBS4, Noor Ul Huda Ramzan, MD5, Mian Uman Anwer, MBBS6, Prasun K.. Jalal, MD7. P5215 - From Bleeding to Healing: Unveiling the Efficacy and Safety of UI-EWD Hemostatic Powder in Gastrointestinal Bleeding - A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Kirk Kerkorian School of Medicine at the University of Nevada Las Vegas, Las Vegas, NV; 2King Edward Medical University, Lahore, Punjab, Pakistan; 3Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 4Quetta Institute of Medical Sciences, Quetta, Balochistan, Pakistan; 5University of Texas Southwestern Medical Center, Dallas, TX; 6Punjab Medical College, Faisalabad, Punjab, Pakistan; 7Baylor College of Medicine, Houston, TX
Introduction: A novel endoscopic hemostatic adhesive powder (UI-EWD) was developed to cope with the high rebleeding rates associated with the current endoscopic hemostatic options for gastrointestinal bleeding. This meta-analysis aimed to assess the safety and effectiveness of UI-EWD hemostatic adhesive powder as a salvage treatment modality for upper and lower gastrointestinal bleeding.
Methods: Electronic databases like PubMed, Cochrane Central and ScienceDirect were searched from inception to January 2025. This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The proportions were pooled with 95 % Confidence Intervals (CI) using R version 4.2.3 and employing the “metaprop” package for the dichotomous outcomes. The primary and secondary outcomes of interest were clinical success, overall rebleeding, early rebleeding, delayed rebleeding, time to rebleeding, mortality and adverse events. The quality assessment was done through the Cochrane Risk of Bias (RoB) 2.0 tool and the Newcastle Ottawa Scale. The risk of publication bias was assessed visually through funnel plots and statistically through Egger’s regression test.
Results: Three studies (including two trials and one cohort) with a total of 211 patients were included in this meta-analysis. The pooled clinical success rate was 98% (95% confidence interval:[85-100 %]; I2 = 0%). The overall rebleeding rate was 11% (95% confidence interval:[0-93%]; I2=11.5% ). The pooled rates of early and delayed rebleeding were 10% (95%CI:[1- 51 %]; I2=70.5%) and 9% (95%CI:[3 - 22%]; I2=0%) respectively.
Discussion: UI-EWD hemostatic adhesive powder demonstrates a high clinical success rate and relatively low overall rebleeding rates, making it an effective salvage treatment for gastrointestinal bleeding. The early and delayed rebleeding rates are manageable, with minimal heterogeneity observed in delayed rebleeding outcomes. These findings suggest UI-EWD is a promising and safe option for managing challenging bleeding cases

Figure: Figure 1: Forest Plots for (A)Clinical Success (B)Early Rebleeding (C)Delayed Rebleeding (D)Overall Rebleeding

Figure: Figure 2: Baseline Characteristics of the Included Studies
Disclosures:
Abu-Bakr Ahmed indicated no relevant financial relationships.
Khadija Mohib indicated no relevant financial relationships.
Zain Ul Abideen indicated no relevant financial relationships.
Muhammad Hassan Waseem indicated no relevant financial relationships.
Sania Aimen indicated no relevant financial relationships.
Noor Ul Huda Ramzan indicated no relevant financial relationships.
Mian Uman Anwer indicated no relevant financial relationships.
Prasun Jalal: AbbVie – Consultant. Gilead Sciences – Consultant.
Abu-Bakr Ahmed, BA1, Khadija Mohib, MD1, Zain Ul Abideen, MBBS2, Muhammad Hassan Waseem, MBBS3, Sania Aimen, MBBS4, Noor Ul Huda Ramzan, MD5, Mian Uman Anwer, MBBS6, Prasun K.. Jalal, MD7. P5215 - From Bleeding to Healing: Unveiling the Efficacy and Safety of UI-EWD Hemostatic Powder in Gastrointestinal Bleeding - A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
