Tuesday Poster Session
Category: GI Bleeding
P5201 - Oral Vonoprazan Is Noninferior to Intravenous Proton Pump Inhibitors for Peptic Ulcer Rebleeding: A Matched Cohort Analysis of Clinical Outcomes
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Saqr Alsakarneh, MD, MS (he/him/his)
Mayo Clinic
Rochester, MN
Presenting Author(s)
Award: ACG Outstanding Research Award in the GI Bleeding Category (Trainee)
Award: ACG Presidential Poster Award
Saqr Alsakarneh, MD, MS1, Mohamad Adam, MD2, Razan Aburumman, MD3, Osama Asad, MD2, Abdulla Massad, MD4, Yazan Abboud, MD5, Yassine Kilani, MD6, Mohammad Bilal, MD, FACG7
1Mayo Clinic, Kansas City, MO; 2University of Missouri - Kansas City School of Medicine, Kansas City, MO; 3Henry Ford Health, Detroit, MI; 4University of Texas Medical Branch, Galveston, TX; 5Rutgers New Jersey Medical School, Newark, NJ; 6Saint Louis University, Saint Louis, MO; 7University of Colorado Anschutz Medical Campus, Denver, CO
Introduction: The American College of Gastroenterology (ACG) guidelines recommend high-dose intravenous proton pump inhibitor (PPI) therapy for 72 hours following successful endoscopic hemostasis of peptic ulcer bleeding. Vonoprazan, a novel potassium-competitive acid blocker (PCAB), is gaining use in acid-related gastrointestinal disorders. This study aimed to compare the efficacy of oral vonoprazan versus intravenous PPIs in preventing peptic ulcer rebleeding using a real-world national database.
Methods: We conducted a retrospective cohort study using the TriNetX research network to identify hospitalized adults (≥18 years) with peptic ulcer bleeding treated with oral vonoprazan or intravenous PPIs. Patients were matched 1:1 using propensity scores based on demographics, race/ethnicity, comorbidities, and medication use (Table 1). The primary outcome was 30-day ulcer rebleeding. Secondary outcomes included readmission rates, emergency department visits, blood transfusion, all-cause mortality, and endoscopic re-intervention at 1, 3, and 6 months. Outcomes were compared using adjusted odds ratios (aOR) with 95% confidence intervals (CIs).
Results: Before matching, vonoprazan users had higher baseline rates of obesity (31.5% vs. 19.6%), ischemic heart disease (44.9% vs. 27.9%), and anticoagulant use (59.1% vs. 39.9%) (all p< 0.001). After matching, the cohorts were well-balanced (mean age 64 years; 50.4% female) (Table 1).
There was no statistically significant difference in 30-day rebleeding (7.9% vs. 12.6%; aOR 0.59; 95% CI: 0.26–1.36; p=0.21) or in emergency visits (aOR 1.69; 95% CI: 0.73–3.87; p=0.21). However, vonoprazan was associated with significantly lower 1-month readmission rates (11.8% vs. 29.1%; aOR 0.33; 95% CI: 0.17–0.63; p=0.001) and at 3 months (18.1% vs. 29.9%; aOR 0.52; 95% CI: 0.29–0.94; p=0.028). No significant differences were observed in blood transfusion rates, endoscopic reintervention, or mortality at any timepoint (Table 2).
Discussion: In this national, propensity-matched cohort study, vonoprazan was noninferior to intravenous PPIs in preventing 30-day peptic ulcer rebleeding following endoscopic hemostasis. Importantly, vonoprazan was associated with significantly lower hospital readmission rates at 1 and 3 months, highlighting its real-world utility and potential cost savings as an oral alternative to IV PPI therapy in peptic ulcer bleeding.
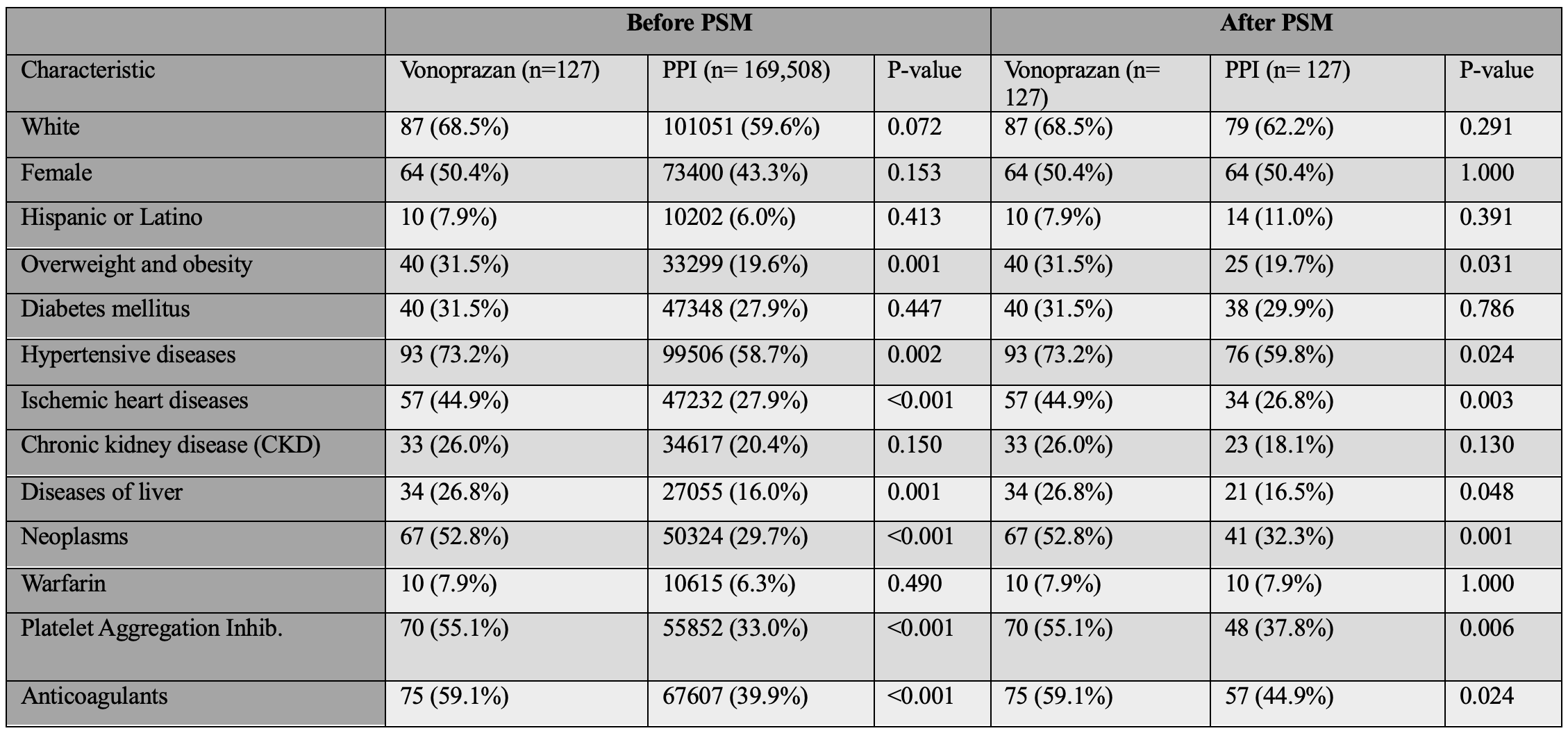
Figure: Table 1. Baseline characteristics of patients treated with vonoprazan versus intravenous proton pump inhibitors (PPIs), before and after 1:1 propensity score matching (PSM).
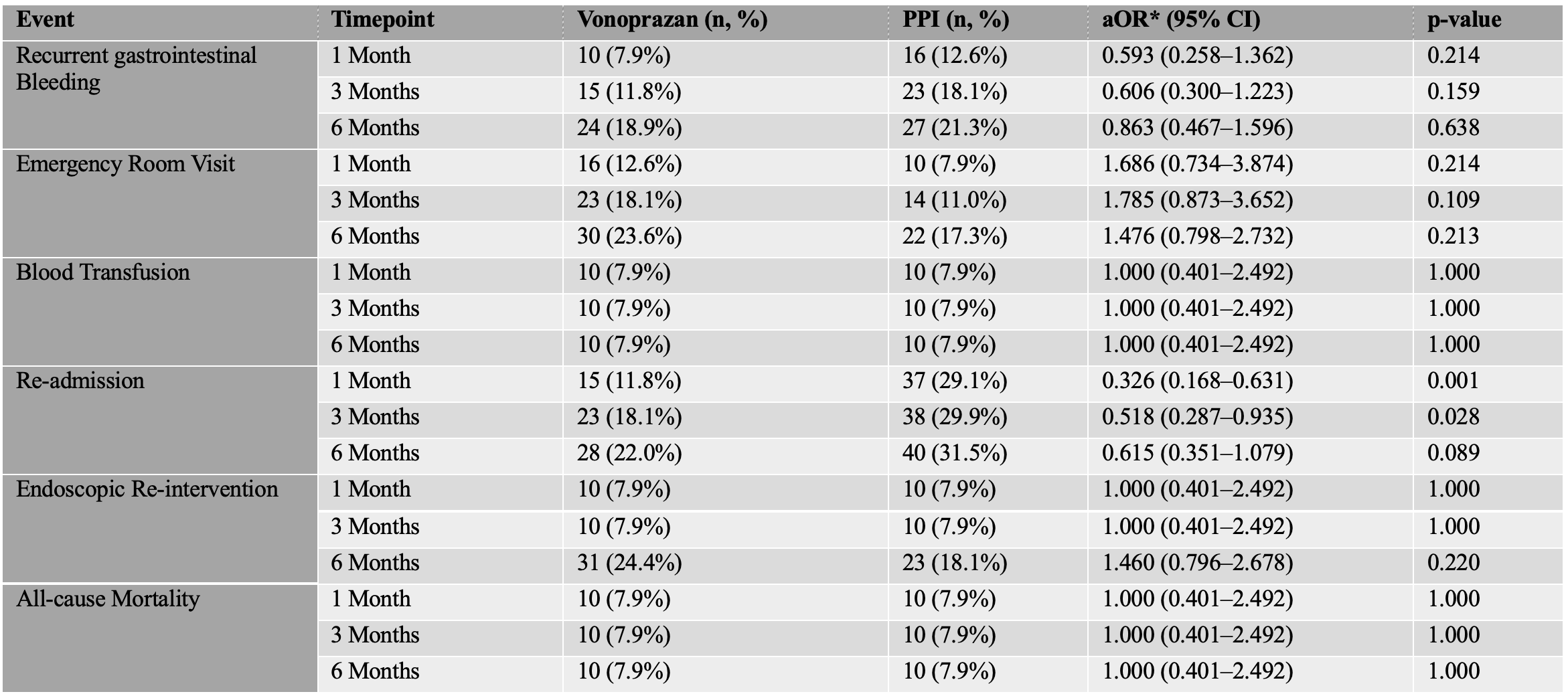
Figure: Table 2: Comparison of Clinical Outcomes Between Vonoprazan and Proton Pump Inhibitors (PPIs) at 1-, 3-, and 6-Months Post-Treatment
*Cohorts were propensity score matched (1:1) for demographics (age and sex), race and ethnicity, comorbid conditions (including obesity, diabetes, hypertension, ischemic heart disease, chronic kidney disease, liver disease, and neoplasms), and medication use (warfarin, platelet aggregation inhibitors, and anticoagulants).
Disclosures:
Saqr Alsakarneh indicated no relevant financial relationships.
Mohamad Adam indicated no relevant financial relationships.
Razan Aburumman indicated no relevant financial relationships.
Osama Asad indicated no relevant financial relationships.
Abdulla Massad indicated no relevant financial relationships.
Yazan Abboud indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Paid speaker. Steris Endoscopy – Consultant.
Saqr Alsakarneh, MD, MS1, Mohamad Adam, MD2, Razan Aburumman, MD3, Osama Asad, MD2, Abdulla Massad, MD4, Yazan Abboud, MD5, Yassine Kilani, MD6, Mohammad Bilal, MD, FACG7. P5201 - Oral Vonoprazan Is Noninferior to Intravenous Proton Pump Inhibitors for Peptic Ulcer Rebleeding: A Matched Cohort Analysis of Clinical Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Award: ACG Presidential Poster Award
Saqr Alsakarneh, MD, MS1, Mohamad Adam, MD2, Razan Aburumman, MD3, Osama Asad, MD2, Abdulla Massad, MD4, Yazan Abboud, MD5, Yassine Kilani, MD6, Mohammad Bilal, MD, FACG7
1Mayo Clinic, Kansas City, MO; 2University of Missouri - Kansas City School of Medicine, Kansas City, MO; 3Henry Ford Health, Detroit, MI; 4University of Texas Medical Branch, Galveston, TX; 5Rutgers New Jersey Medical School, Newark, NJ; 6Saint Louis University, Saint Louis, MO; 7University of Colorado Anschutz Medical Campus, Denver, CO
Introduction: The American College of Gastroenterology (ACG) guidelines recommend high-dose intravenous proton pump inhibitor (PPI) therapy for 72 hours following successful endoscopic hemostasis of peptic ulcer bleeding. Vonoprazan, a novel potassium-competitive acid blocker (PCAB), is gaining use in acid-related gastrointestinal disorders. This study aimed to compare the efficacy of oral vonoprazan versus intravenous PPIs in preventing peptic ulcer rebleeding using a real-world national database.
Methods: We conducted a retrospective cohort study using the TriNetX research network to identify hospitalized adults (≥18 years) with peptic ulcer bleeding treated with oral vonoprazan or intravenous PPIs. Patients were matched 1:1 using propensity scores based on demographics, race/ethnicity, comorbidities, and medication use (Table 1). The primary outcome was 30-day ulcer rebleeding. Secondary outcomes included readmission rates, emergency department visits, blood transfusion, all-cause mortality, and endoscopic re-intervention at 1, 3, and 6 months. Outcomes were compared using adjusted odds ratios (aOR) with 95% confidence intervals (CIs).
Results: Before matching, vonoprazan users had higher baseline rates of obesity (31.5% vs. 19.6%), ischemic heart disease (44.9% vs. 27.9%), and anticoagulant use (59.1% vs. 39.9%) (all p< 0.001). After matching, the cohorts were well-balanced (mean age 64 years; 50.4% female) (Table 1).
There was no statistically significant difference in 30-day rebleeding (7.9% vs. 12.6%; aOR 0.59; 95% CI: 0.26–1.36; p=0.21) or in emergency visits (aOR 1.69; 95% CI: 0.73–3.87; p=0.21). However, vonoprazan was associated with significantly lower 1-month readmission rates (11.8% vs. 29.1%; aOR 0.33; 95% CI: 0.17–0.63; p=0.001) and at 3 months (18.1% vs. 29.9%; aOR 0.52; 95% CI: 0.29–0.94; p=0.028). No significant differences were observed in blood transfusion rates, endoscopic reintervention, or mortality at any timepoint (Table 2).
Discussion: In this national, propensity-matched cohort study, vonoprazan was noninferior to intravenous PPIs in preventing 30-day peptic ulcer rebleeding following endoscopic hemostasis. Importantly, vonoprazan was associated with significantly lower hospital readmission rates at 1 and 3 months, highlighting its real-world utility and potential cost savings as an oral alternative to IV PPI therapy in peptic ulcer bleeding.

Figure: Table 1. Baseline characteristics of patients treated with vonoprazan versus intravenous proton pump inhibitors (PPIs), before and after 1:1 propensity score matching (PSM).

Figure: Table 2: Comparison of Clinical Outcomes Between Vonoprazan and Proton Pump Inhibitors (PPIs) at 1-, 3-, and 6-Months Post-Treatment
*Cohorts were propensity score matched (1:1) for demographics (age and sex), race and ethnicity, comorbid conditions (including obesity, diabetes, hypertension, ischemic heart disease, chronic kidney disease, liver disease, and neoplasms), and medication use (warfarin, platelet aggregation inhibitors, and anticoagulants).
Disclosures:
Saqr Alsakarneh indicated no relevant financial relationships.
Mohamad Adam indicated no relevant financial relationships.
Razan Aburumman indicated no relevant financial relationships.
Osama Asad indicated no relevant financial relationships.
Abdulla Massad indicated no relevant financial relationships.
Yazan Abboud indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Paid speaker. Steris Endoscopy – Consultant.
Saqr Alsakarneh, MD, MS1, Mohamad Adam, MD2, Razan Aburumman, MD3, Osama Asad, MD2, Abdulla Massad, MD4, Yazan Abboud, MD5, Yassine Kilani, MD6, Mohammad Bilal, MD, FACG7. P5201 - Oral Vonoprazan Is Noninferior to Intravenous Proton Pump Inhibitors for Peptic Ulcer Rebleeding: A Matched Cohort Analysis of Clinical Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


