Tuesday Poster Session
Category: General Endoscopy
P5131 - Examining the Impact of Glucagon-Like Peptide-1 Receptor Agonists on Residual Gastric Contents During Upper Endoscopy: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Emeka S. Obi, MBBS (he/him/his)
One Brooklyn Health-Brookdale University Hospital Medical Center
Brooklyn, NY
Presenting Author(s)
Emeka S. Obi, MBBS1, Hezborn Magacha, MD2, Adedeji Adenusi, MD, MPH3, Olanrewaju Adeniran, MD4, Onyinyechi Okam, MD5, Anderson Ikeokwu, MD6, Winifred Iklaki, MD7, Emmanuel Arji, MD, MPH8, Chidiebele Omaliko, MD1
1One Brooklyn Health-Brookdale University Hospital Medical Center, Brooklyn, NY; 2East Tennessee State University, Johnson City, TN; 3One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 4West Virginia University Morgantown, Morgantown, WV; 5Texas Tech University Health Sciences Center Amarillo, Amarillo, TX; 6Department of Medicine, Kingston Public Hospital, Jamaica, Jamaica, NY; 7University of Missouri Columbia Hospital, Columbia, MO; 8Woodhull Medical Center, Brooklyn, NY
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) delay gastric emptying, affecting glucose metabolism, appetite, and weight management in patients with type 2 diabetes. Individuals using GLP-1 RAs may have increased residual gastric contents, raising the risk of aspiration during endoscopic procedures. Previous studies on this association have shown mixed results. This systematic review and meta-analysis aimed to evaluate the relationship between GLP-1 RA use and residual gastric contents during upper endoscopy.
Methods: We searched PubMed, EMBASE, Google Scholar, Web of Science, and the Cochrane Library through May 2024 for studies assessing GLP-1 RA use and gastric residuals during endoscopy. Reference lists of relevant articles were also reviewed. Both fixed-effects and random-effects models were used for the meta-analysis.
Results: Of 46 studies screened, 6 cohorts met the inclusion criteria. One was excluded due to incompatible outcome measures and missing data. The final analysis included 5 cohorts with a total of 1,307 participants, of whom 449 (34.4%) were GLP-1 RA users and 858 (65.6%) were non-users. GLP-1 RA users had significantly higher odds of increased gastric residual contents (OR 2.53, 95% CI: 1.63–3.92; p < 0.001). Significant heterogeneity was observed (p < 0.0001). Egger's test indicated no publication bias (p = 0.94).
Discussion: This meta-analysis demonstrates that GLP-1 RAs significantly impact gastric motility and are associated with increased gastric contents in fasting patients undergoing upper endoscopy. However, data on the associated aspiration risk and effects on endoscopic visualization quality remain limited. Further research is warranted to explore these clinical implications.
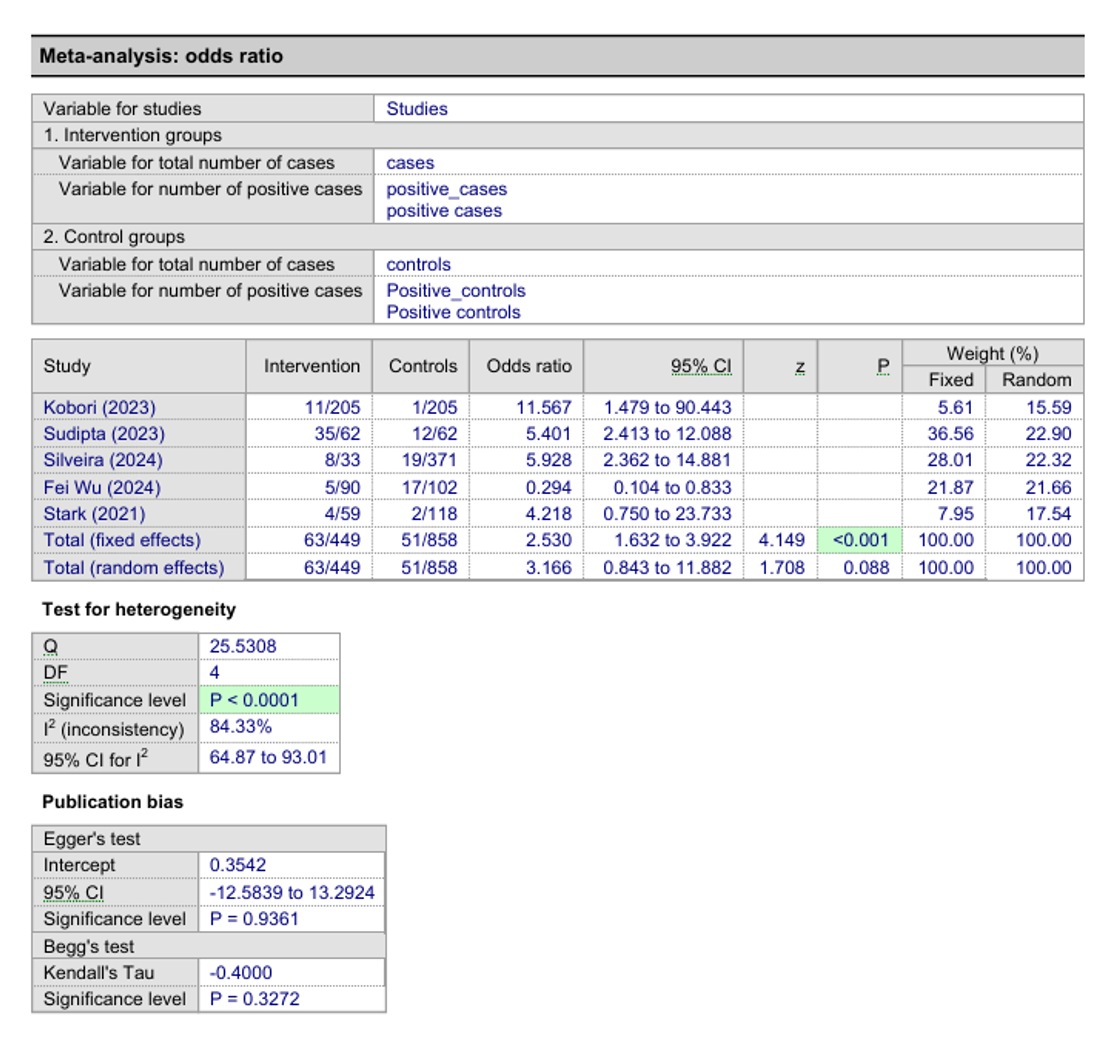
Figure: Meta Analysis Odds Ratio | Test for Heterogeneity | Publication Bias
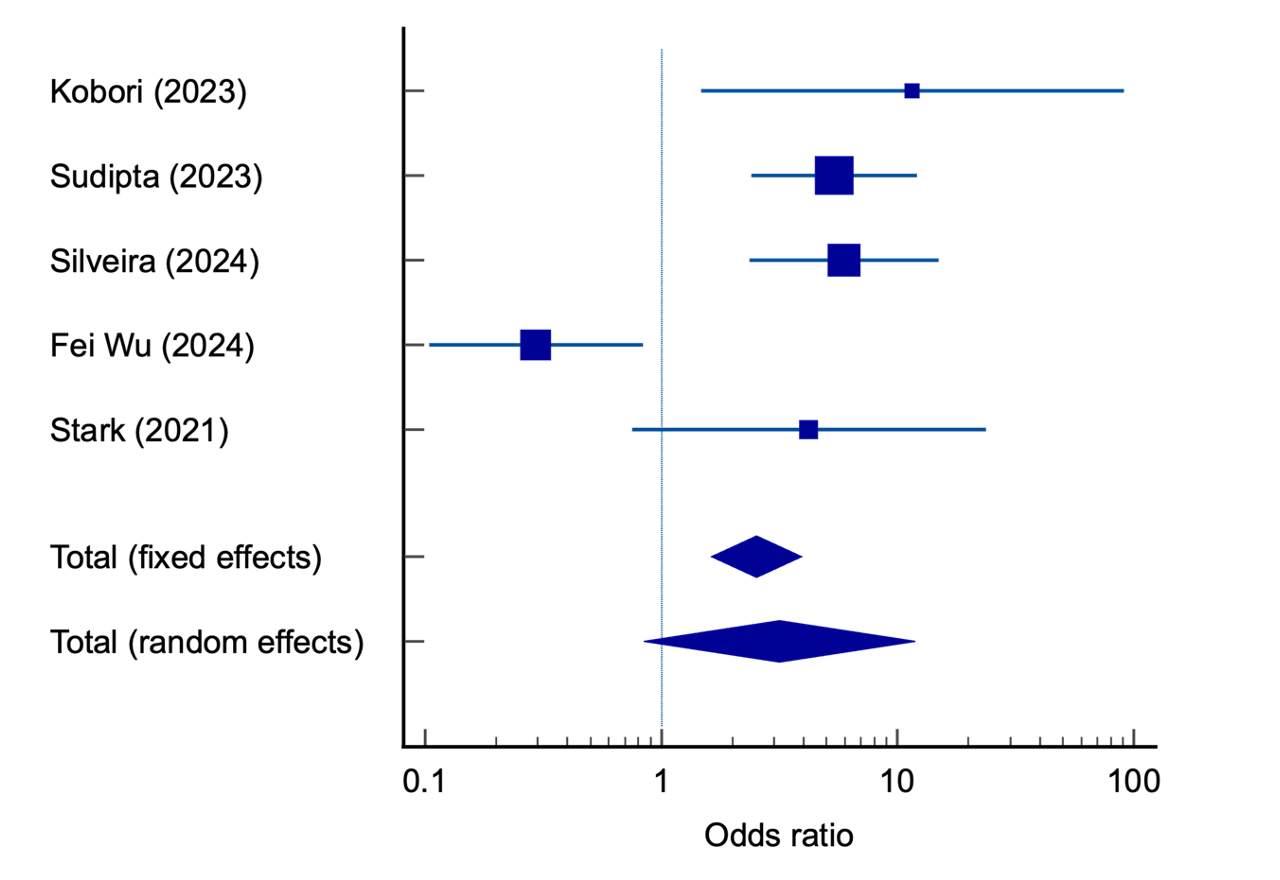
Figure: Forest Plot
Disclosures:
Emeka Obi indicated no relevant financial relationships.
Hezborn Magacha indicated no relevant financial relationships.
Adedeji Adenusi indicated no relevant financial relationships.
Olanrewaju Adeniran indicated no relevant financial relationships.
Onyinyechi Okam indicated no relevant financial relationships.
Anderson Ikeokwu indicated no relevant financial relationships.
Winifred Iklaki indicated no relevant financial relationships.
Emmanuel Arji indicated no relevant financial relationships.
Chidiebele Omaliko indicated no relevant financial relationships.
Emeka S. Obi, MBBS1, Hezborn Magacha, MD2, Adedeji Adenusi, MD, MPH3, Olanrewaju Adeniran, MD4, Onyinyechi Okam, MD5, Anderson Ikeokwu, MD6, Winifred Iklaki, MD7, Emmanuel Arji, MD, MPH8, Chidiebele Omaliko, MD1. P5131 - Examining the Impact of Glucagon-Like Peptide-1 Receptor Agonists on Residual Gastric Contents During Upper Endoscopy: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1One Brooklyn Health-Brookdale University Hospital Medical Center, Brooklyn, NY; 2East Tennessee State University, Johnson City, TN; 3One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 4West Virginia University Morgantown, Morgantown, WV; 5Texas Tech University Health Sciences Center Amarillo, Amarillo, TX; 6Department of Medicine, Kingston Public Hospital, Jamaica, Jamaica, NY; 7University of Missouri Columbia Hospital, Columbia, MO; 8Woodhull Medical Center, Brooklyn, NY
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) delay gastric emptying, affecting glucose metabolism, appetite, and weight management in patients with type 2 diabetes. Individuals using GLP-1 RAs may have increased residual gastric contents, raising the risk of aspiration during endoscopic procedures. Previous studies on this association have shown mixed results. This systematic review and meta-analysis aimed to evaluate the relationship between GLP-1 RA use and residual gastric contents during upper endoscopy.
Methods: We searched PubMed, EMBASE, Google Scholar, Web of Science, and the Cochrane Library through May 2024 for studies assessing GLP-1 RA use and gastric residuals during endoscopy. Reference lists of relevant articles were also reviewed. Both fixed-effects and random-effects models were used for the meta-analysis.
Results: Of 46 studies screened, 6 cohorts met the inclusion criteria. One was excluded due to incompatible outcome measures and missing data. The final analysis included 5 cohorts with a total of 1,307 participants, of whom 449 (34.4%) were GLP-1 RA users and 858 (65.6%) were non-users. GLP-1 RA users had significantly higher odds of increased gastric residual contents (OR 2.53, 95% CI: 1.63–3.92; p < 0.001). Significant heterogeneity was observed (p < 0.0001). Egger's test indicated no publication bias (p = 0.94).
Discussion: This meta-analysis demonstrates that GLP-1 RAs significantly impact gastric motility and are associated with increased gastric contents in fasting patients undergoing upper endoscopy. However, data on the associated aspiration risk and effects on endoscopic visualization quality remain limited. Further research is warranted to explore these clinical implications.

Figure: Meta Analysis Odds Ratio | Test for Heterogeneity | Publication Bias

Figure: Forest Plot
Disclosures:
Emeka Obi indicated no relevant financial relationships.
Hezborn Magacha indicated no relevant financial relationships.
Adedeji Adenusi indicated no relevant financial relationships.
Olanrewaju Adeniran indicated no relevant financial relationships.
Onyinyechi Okam indicated no relevant financial relationships.
Anderson Ikeokwu indicated no relevant financial relationships.
Winifred Iklaki indicated no relevant financial relationships.
Emmanuel Arji indicated no relevant financial relationships.
Chidiebele Omaliko indicated no relevant financial relationships.
Emeka S. Obi, MBBS1, Hezborn Magacha, MD2, Adedeji Adenusi, MD, MPH3, Olanrewaju Adeniran, MD4, Onyinyechi Okam, MD5, Anderson Ikeokwu, MD6, Winifred Iklaki, MD7, Emmanuel Arji, MD, MPH8, Chidiebele Omaliko, MD1. P5131 - Examining the Impact of Glucagon-Like Peptide-1 Receptor Agonists on Residual Gastric Contents During Upper Endoscopy: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
