Tuesday Poster Session
Category: Esophagus
P4920 - Evaluating Publication Imbalances and Key Characteristics in Eosinophilic Esophagitis Clinical Trials: Patterns and Trends
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Malik Zdouq, BS
Keck School of Medicine of the University of Southern California
Anaheim, CA
Presenting Author(s)
Malik Zdouq, BS1, Ibrahim Abboud, BS2, Hasson Abdel-Jaber, BS3, Eyad Alsalek, 4, Anton Abboud, BS5, Anisa Shaker, MD6, Edy Soffer, MD6
1Keck School of Medicine of the University of Southern California, Anaheim, CA; 2University of California Riverside School of Medicine, Corona, CA; 3University of California Riverside School of Medicine, Anaheim, CA; 4University of California Riverside School of Medicine, Riverside, CA; 5University of California Riverside, Corona, CA; 6Keck School of Medicine of the University of Southern California, Los Angeles, CA
Introduction: Clinical trials publication discrepancies distort the perceived effectiveness of treatments by placing greater emphasis on studies with positive outcomes while underrepresenting negative findings. This study examines patterns in EoE clinical trials (CT), as their characteristics have not been adequately investigated.
Methods: Utilizing ClinicalTrials, EoE-registered CT were categorized. To assess publication status, a completion date was set, allowing a 3-year window for publication. We searched for trial publications on ClinicalTrials, PubMed, and Google Scholar. Trials that were deemed statistically significant and achieved their objective were classified as positive studies. For statistical analysis, we performed Fisher’s exact test, t-test, and chi-square tests.
Results: There were 196 EoE studies, the majority being interventional (64.80%), of which the majority were in the late phase (59.06%, p< 0.001) and focused on drug treatment (58.27%). Interventional CTs centered on adults (54.33%), while observational CTs included both adults and children (57.97%), p=0.013. Most trials were led by Male PIs (82.65%) with an MD (76.02%). The majority of CTs were performed in the US (76.38% interventional and 71.01% observational, p=0.411) and were non-industry funded (64.57% interventional and 92.75% observational, respectively, p< 0.0001). Industry-funded studies were mostly interventional. Interventional trials had a higher completion rate (54.33% vs 43.48%, p=0.001). Of the 78 completed trials, 56 (71.79%) were published, of which the majority were interventional (75.00%, p=0.004), in the late phase (51.79%, p=0.02), and domestic (83.93%, p=0.822). Among the 56 published trials, 35 (62.5%) had positive outcomes, and 21 (37.5%) had negative outcomes (p=0.205). Of the publications with positive outcomes, similar percentages were interventional vs observational (59.52% vs 71.43%, p=0.158).
Discussion: The majority of EoE clinical trials involve adults and were not industry-sponsored. Most industry studies were interventional. Most published trials were led by male MD PIs, reflecting gender disparities in leadership. Finally, the majority of published trials (interventional or observational) report positive results.
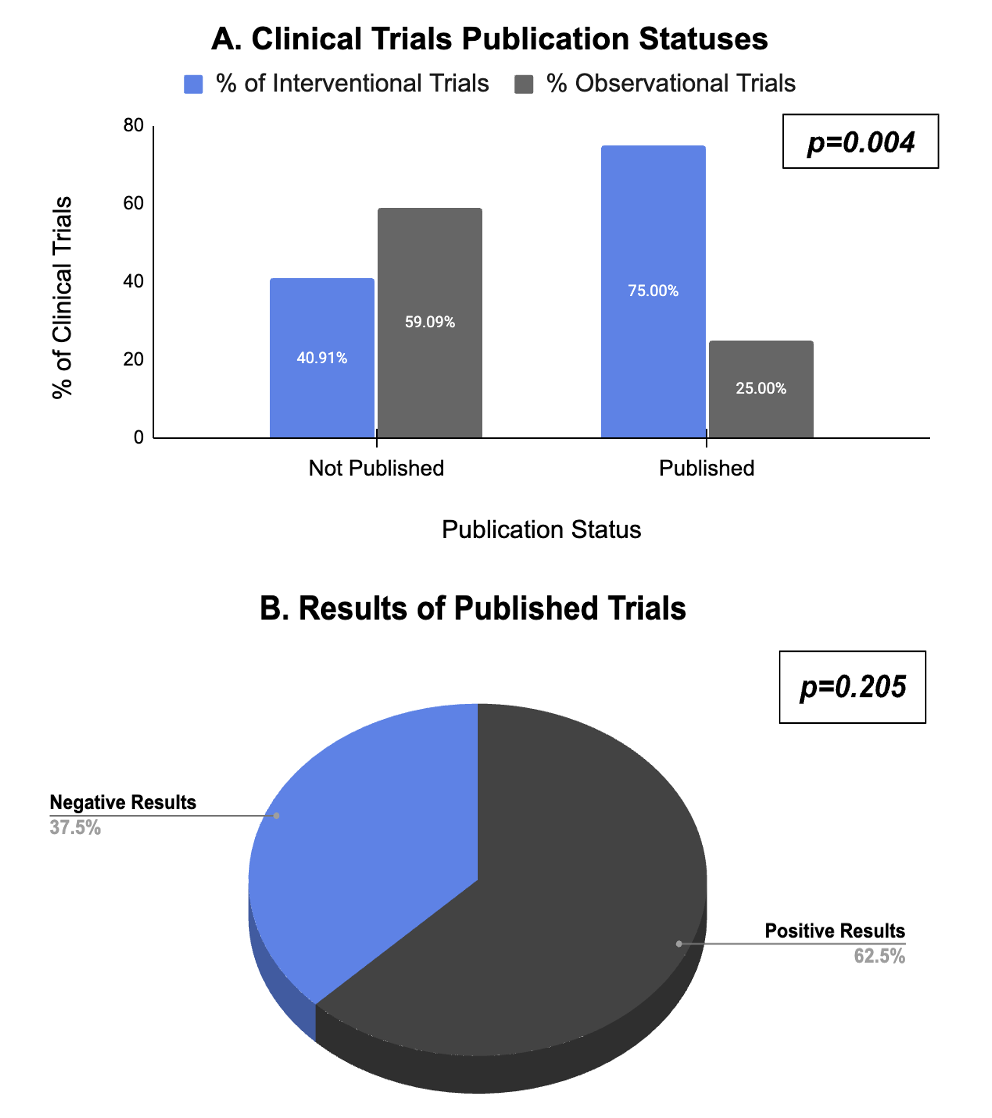
Figure: Figure 1. A) Publication Statuses of Interventional and Observational Completed Clinical Trials. B) Results of All Completed Published Clinical Trials (Positive vs. Negative).
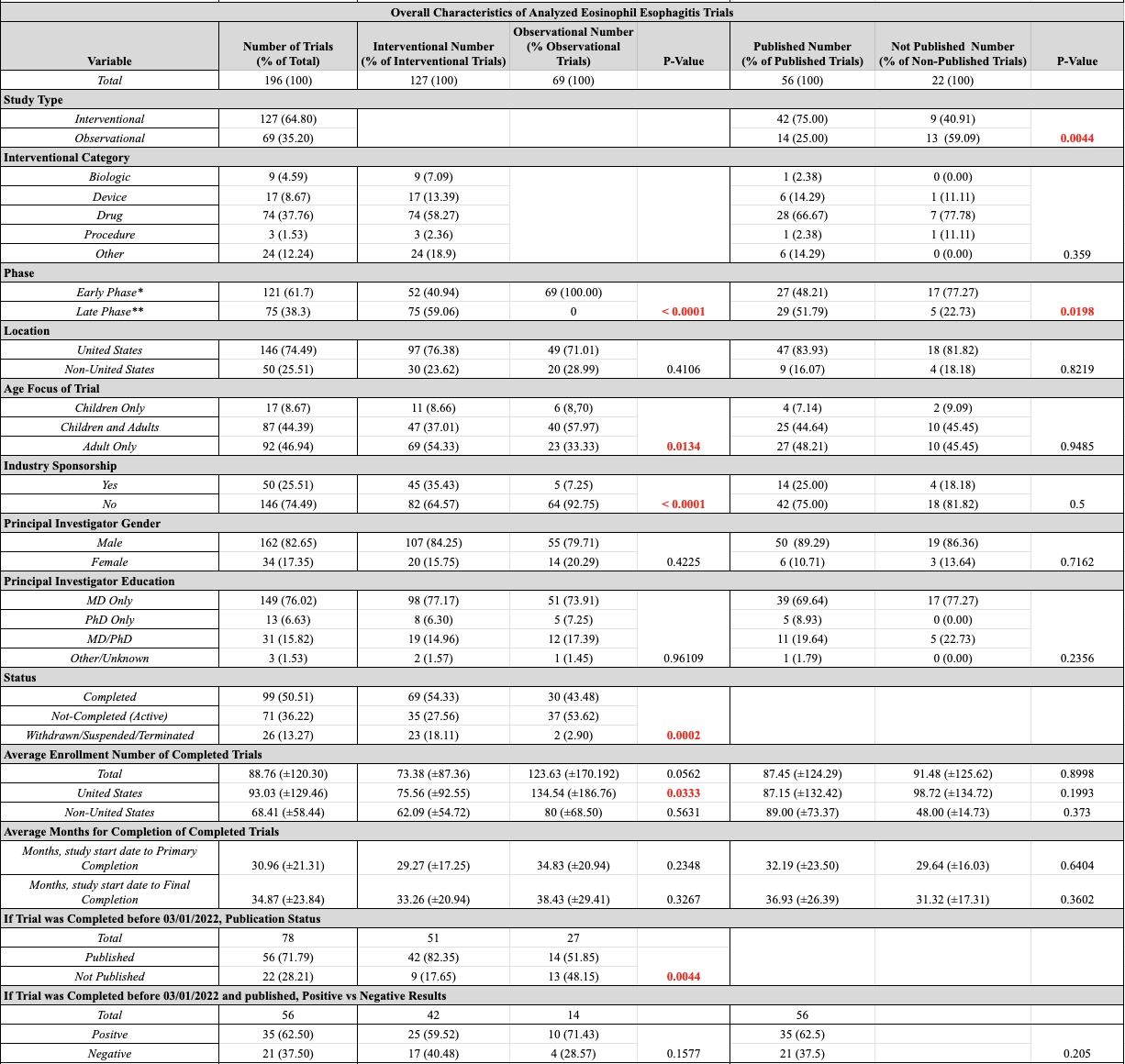
Figure: Table 1: Characteristics of Analyzed Eosinophil Esophagitis Clinical Trials (*Early Phase: Phase 1 Trial or Uncategorized
**Late Phase: Phase 2/3, Phase 3, and Phase 4 Trials.)
Disclosures:
Malik Zdouq indicated no relevant financial relationships.
Ibrahim Abboud indicated no relevant financial relationships.
Hasson Abdel-Jaber indicated no relevant financial relationships.
Eyad Alsalek indicated no relevant financial relationships.
Anton Abboud indicated no relevant financial relationships.
Anisa Shaker indicated no relevant financial relationships.
Edy Soffer indicated no relevant financial relationships.
Malik Zdouq, BS1, Ibrahim Abboud, BS2, Hasson Abdel-Jaber, BS3, Eyad Alsalek, 4, Anton Abboud, BS5, Anisa Shaker, MD6, Edy Soffer, MD6. P4920 - Evaluating Publication Imbalances and Key Characteristics in Eosinophilic Esophagitis Clinical Trials: Patterns and Trends, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Keck School of Medicine of the University of Southern California, Anaheim, CA; 2University of California Riverside School of Medicine, Corona, CA; 3University of California Riverside School of Medicine, Anaheim, CA; 4University of California Riverside School of Medicine, Riverside, CA; 5University of California Riverside, Corona, CA; 6Keck School of Medicine of the University of Southern California, Los Angeles, CA
Introduction: Clinical trials publication discrepancies distort the perceived effectiveness of treatments by placing greater emphasis on studies with positive outcomes while underrepresenting negative findings. This study examines patterns in EoE clinical trials (CT), as their characteristics have not been adequately investigated.
Methods: Utilizing ClinicalTrials, EoE-registered CT were categorized. To assess publication status, a completion date was set, allowing a 3-year window for publication. We searched for trial publications on ClinicalTrials, PubMed, and Google Scholar. Trials that were deemed statistically significant and achieved their objective were classified as positive studies. For statistical analysis, we performed Fisher’s exact test, t-test, and chi-square tests.
Results: There were 196 EoE studies, the majority being interventional (64.80%), of which the majority were in the late phase (59.06%, p< 0.001) and focused on drug treatment (58.27%). Interventional CTs centered on adults (54.33%), while observational CTs included both adults and children (57.97%), p=0.013. Most trials were led by Male PIs (82.65%) with an MD (76.02%). The majority of CTs were performed in the US (76.38% interventional and 71.01% observational, p=0.411) and were non-industry funded (64.57% interventional and 92.75% observational, respectively, p< 0.0001). Industry-funded studies were mostly interventional. Interventional trials had a higher completion rate (54.33% vs 43.48%, p=0.001). Of the 78 completed trials, 56 (71.79%) were published, of which the majority were interventional (75.00%, p=0.004), in the late phase (51.79%, p=0.02), and domestic (83.93%, p=0.822). Among the 56 published trials, 35 (62.5%) had positive outcomes, and 21 (37.5%) had negative outcomes (p=0.205). Of the publications with positive outcomes, similar percentages were interventional vs observational (59.52% vs 71.43%, p=0.158).
Discussion: The majority of EoE clinical trials involve adults and were not industry-sponsored. Most industry studies were interventional. Most published trials were led by male MD PIs, reflecting gender disparities in leadership. Finally, the majority of published trials (interventional or observational) report positive results.

Figure: Figure 1. A) Publication Statuses of Interventional and Observational Completed Clinical Trials. B) Results of All Completed Published Clinical Trials (Positive vs. Negative).

Figure: Table 1: Characteristics of Analyzed Eosinophil Esophagitis Clinical Trials (*Early Phase: Phase 1 Trial or Uncategorized
**Late Phase: Phase 2/3, Phase 3, and Phase 4 Trials.)
Disclosures:
Malik Zdouq indicated no relevant financial relationships.
Ibrahim Abboud indicated no relevant financial relationships.
Hasson Abdel-Jaber indicated no relevant financial relationships.
Eyad Alsalek indicated no relevant financial relationships.
Anton Abboud indicated no relevant financial relationships.
Anisa Shaker indicated no relevant financial relationships.
Edy Soffer indicated no relevant financial relationships.
Malik Zdouq, BS1, Ibrahim Abboud, BS2, Hasson Abdel-Jaber, BS3, Eyad Alsalek, 4, Anton Abboud, BS5, Anisa Shaker, MD6, Edy Soffer, MD6. P4920 - Evaluating Publication Imbalances and Key Characteristics in Eosinophilic Esophagitis Clinical Trials: Patterns and Trends, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
