Tuesday Poster Session
Category: Esophagus
P4919 - Diagnostic and Therapeutic Monitoring of Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis of Non-invasive Biomarkers
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- NV
Naga Praneeth Vakkalagadda, MD
University of Alabama at Birmingham Heersink School of Medicine
Montgomery, AL
Presenting Author(s)
Naga Praneeth Vakkalagadda, MD1, Yashaswi Guntupalli, MBBS2, Rithish Nimmagadda, MBBS3, Vineeth Potluri, MD4, Nayanika Tummala, MD5, Ravi Medarametla, MD6, Jahnavi Udaikumar, MD7
1University of Alabama at Birmingham Heersink School of Medicine, Montgomery, AL; 2Sri Padmavathi Medical College for Women, SVIMS, Tirupati, Tirupati, Andhra Pradesh, India; 3One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 4Cleveland Clinic, Cleveland, OH; 5St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY; 6Mamata Medical College, Khammam, Telangana, India; 7NYU Grossman School of Medicine, Department of Medicine, New York, NY
Introduction: A chronic, immune-mediated esophageal condition, eosinophilic oesophagitis (EoE), calls for repeated disease activity and therapeutic response evaluation. Although endoscopy with biopsy is still the diagnostic gold standard, its invasibility and cost restrict frequent use. Noninvasive biomarkers, such as eosinophil-derived neurotoxin (EDN), eosinophil cationic protein (ECP), serum IgG4, periostin, mast cell tryptase (MCT), and exhaled nitric oxide (FeNO), have been alternatively proposed as diagnostic and monitoring tools. Our objective was to evaluate their clinical value and diagnostic accuracy in tracking treatment response and identifying histologically active EoE.
Methods: We systematically searched PubMed, EMBASE, and Cochrane CENTRAL from inception through April 2025. We included clinical trials comparing noninvasive biomarkers' value in EoE against histologic reference criteria (≥ 15 eosinophils per high‐power field [eos/hpf]). Inverse variance weighting technique was used to pool the data, and random effects model was applied to explain heterogeneity among the studies.
Results: Although sixteen studies met the inclusion criteria, only three were randomized controlled trials (RCTs) evaluating active intervention versus placebo for changes in biomarker levels and thus met the specific requirements for inclusion in our analysis. Mean percentage change for Eotaxin-3 between intervention and placebo is –51.67 (95% CI: –60.01 to –43.33; P< 0.0001; I²=0%), and for TARC(CCL-17) between intervention and placebo is –22.82 (95% CI: –30.31 to –15.34; P< 0.0001; I²=0%), indicating that intervention significantly lowered Eotaxin-3 and TARC(CCL-17) levels compared to placebo. There is no heterogeneity among included studies, indicating consistent treatment effects among included studies.
Discussion: Several noninvasive biomarkers, especially eotaxin-3 and TARC/CCL-17, showed clinically and statistically significant drops with effective treatment. This supports their use in tracking disease activity in EoE. While these biomarkers do not yet replace endoscopy, they offer valuable adjunctive tools to assess treatment response and reduce procedural burden in clinical practice and will need further studies to evaluate their use.
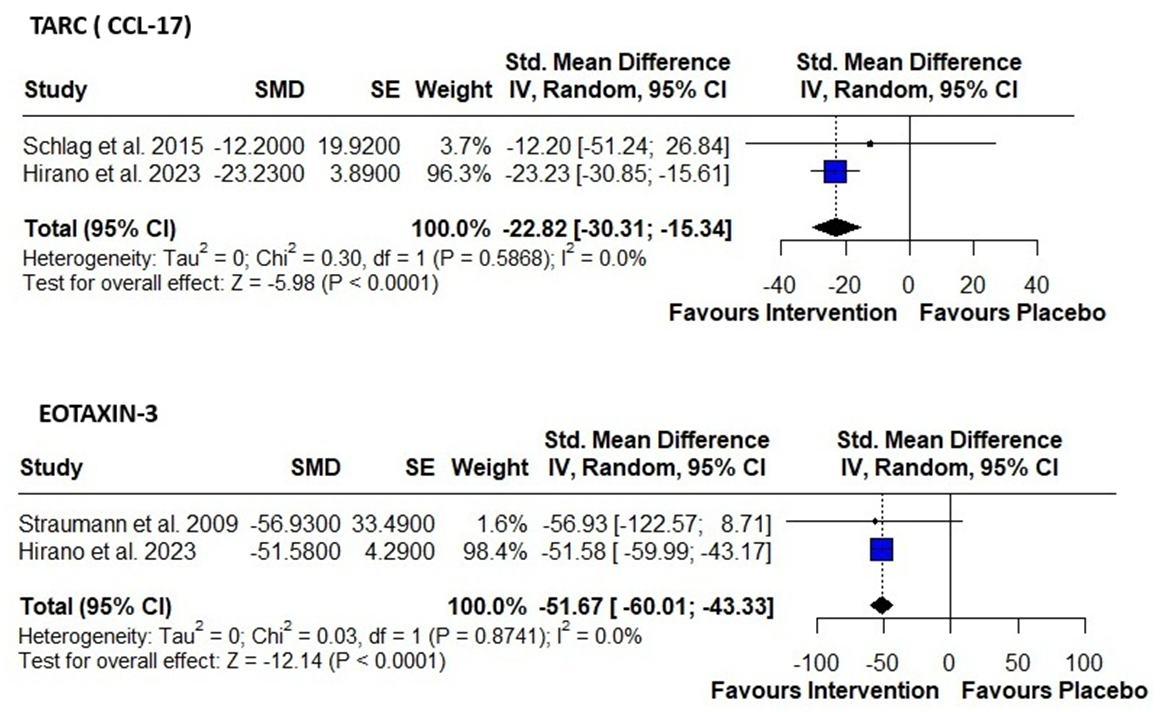
Figure: Table 1: Forest plots showing standardized mean differences (SMD) in percent change of biomarker levels between intervention and placebo groups in patients with eosinophilic esophagitis (EoE).
Disclosures:
Naga Praneeth Vakkalagadda indicated no relevant financial relationships.
Yashaswi Guntupalli indicated no relevant financial relationships.
Rithish Nimmagadda indicated no relevant financial relationships.
Vineeth Potluri indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Ravi Medarametla indicated no relevant financial relationships.
Jahnavi Udaikumar indicated no relevant financial relationships.
Naga Praneeth Vakkalagadda, MD1, Yashaswi Guntupalli, MBBS2, Rithish Nimmagadda, MBBS3, Vineeth Potluri, MD4, Nayanika Tummala, MD5, Ravi Medarametla, MD6, Jahnavi Udaikumar, MD7. P4919 - Diagnostic and Therapeutic Monitoring of Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis of Non-invasive Biomarkers, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Alabama at Birmingham Heersink School of Medicine, Montgomery, AL; 2Sri Padmavathi Medical College for Women, SVIMS, Tirupati, Tirupati, Andhra Pradesh, India; 3One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 4Cleveland Clinic, Cleveland, OH; 5St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY; 6Mamata Medical College, Khammam, Telangana, India; 7NYU Grossman School of Medicine, Department of Medicine, New York, NY
Introduction: A chronic, immune-mediated esophageal condition, eosinophilic oesophagitis (EoE), calls for repeated disease activity and therapeutic response evaluation. Although endoscopy with biopsy is still the diagnostic gold standard, its invasibility and cost restrict frequent use. Noninvasive biomarkers, such as eosinophil-derived neurotoxin (EDN), eosinophil cationic protein (ECP), serum IgG4, periostin, mast cell tryptase (MCT), and exhaled nitric oxide (FeNO), have been alternatively proposed as diagnostic and monitoring tools. Our objective was to evaluate their clinical value and diagnostic accuracy in tracking treatment response and identifying histologically active EoE.
Methods: We systematically searched PubMed, EMBASE, and Cochrane CENTRAL from inception through April 2025. We included clinical trials comparing noninvasive biomarkers' value in EoE against histologic reference criteria (≥ 15 eosinophils per high‐power field [eos/hpf]). Inverse variance weighting technique was used to pool the data, and random effects model was applied to explain heterogeneity among the studies.
Results: Although sixteen studies met the inclusion criteria, only three were randomized controlled trials (RCTs) evaluating active intervention versus placebo for changes in biomarker levels and thus met the specific requirements for inclusion in our analysis. Mean percentage change for Eotaxin-3 between intervention and placebo is –51.67 (95% CI: –60.01 to –43.33; P< 0.0001; I²=0%), and for TARC(CCL-17) between intervention and placebo is –22.82 (95% CI: –30.31 to –15.34; P< 0.0001; I²=0%), indicating that intervention significantly lowered Eotaxin-3 and TARC(CCL-17) levels compared to placebo. There is no heterogeneity among included studies, indicating consistent treatment effects among included studies.
Discussion: Several noninvasive biomarkers, especially eotaxin-3 and TARC/CCL-17, showed clinically and statistically significant drops with effective treatment. This supports their use in tracking disease activity in EoE. While these biomarkers do not yet replace endoscopy, they offer valuable adjunctive tools to assess treatment response and reduce procedural burden in clinical practice and will need further studies to evaluate their use.

Figure: Table 1: Forest plots showing standardized mean differences (SMD) in percent change of biomarker levels between intervention and placebo groups in patients with eosinophilic esophagitis (EoE).
Disclosures:
Naga Praneeth Vakkalagadda indicated no relevant financial relationships.
Yashaswi Guntupalli indicated no relevant financial relationships.
Rithish Nimmagadda indicated no relevant financial relationships.
Vineeth Potluri indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Ravi Medarametla indicated no relevant financial relationships.
Jahnavi Udaikumar indicated no relevant financial relationships.
Naga Praneeth Vakkalagadda, MD1, Yashaswi Guntupalli, MBBS2, Rithish Nimmagadda, MBBS3, Vineeth Potluri, MD4, Nayanika Tummala, MD5, Ravi Medarametla, MD6, Jahnavi Udaikumar, MD7. P4919 - Diagnostic and Therapeutic Monitoring of Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis of Non-invasive Biomarkers, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
