Tuesday Poster Session
Category: Esophagus
P4909 - Safety and Efficacy of Eluxadoline for Irritable Bowel Syndrome (Diarrhea Predominant): Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- VB
Vinay Bellur
Ramaiah medical college
Bangalore, Karnataka, India
Presenting Author(s)
Shradha Chervittara Karaveetil, 1, Vinay Chandramouli Bellur, 2, Ananya Prasad, 1, Omar Oudit, DO3, Allama Prabhu N S, 4, Rishikesh R. Magaji, 5, Trisha Chandra Mohan, 5, Aashray Alva, 1, Keerthi Balaji Babu Naidu, 2, Aryan Gupta, 6, Pavan Kumara Kasam Shiva, 6, Deepika A, 6, A. Shree Charan, 7, Vibhav MS, 6, Druvadeep Srinivas, 8, Vardhini Ganesh Iyer, 5, Sravani Bhavanam, 9
1ramaiah medical college, Bangalore, Karnataka, India; 2Ramaiah medical college, Bangalore, Karnataka, India; 3Brookdale University Hospital Medical Center, Brooklyn, NY; 4Bangalore Medical College and Research Institute, Bangalore, Karnataka, India; 5BGS Global Institute of Medical Sciences, Bangalore, Karnataka, India; 6bangalore medical college and research institute, Bangalore, Karnataka, India; 7Ramaiah Medical College, Bangalore, Karnataka, India; 8rajarajeshwari medical college & hospital, Bangalore, Karnataka, India; 9Brookdale University Hospital Medical Center, Bangalore, Karnataka, India
Introduction: Irritable Bowel Syndrome with Diarrhea (IBS-D) is a chronic functional disorder marked by frequent loose stools, urgency, and abdominal discomfort, significantly impairing quality of life. Eluxadoline, a mixed μ- and κ-opioid receptor agonist and δ-opioid antagonist, has emerged as a targeted treatment for IBS-D. This meta-analysis evaluates the efficacy and safety of Eluxadoline in reducing diarrheal symptoms and improving overall symptom control in IBS-D patients.
Methods: The review conducted follows the PRISMA guidelines and major medical databases, which include PUBMED, Google Scholar and Science-Direct, were extensively searched using a comprehensive search term to identify and retrieve available articles. The articles included the assessment of outcomes following Eluxadoline therapy for Irritable bowel syndrome.
The data was analysed using the Meta, Metadata and the Metafor packages of R Studio. The Efficacy of Eluxadoline therapy was assessed by estimating the Odds ratio of the primary end point efficacy between the two groups. The QOL was assessed through the mean change in IBS-Quality of Life score. The Mantel-Haenszel method and the Inverse variance method were utilised to analyse the odds ratio and risk ratio. The I^2 test was used to assess the heterogeneity of the studies.
Results: The review includes a total of 7 articles that assessed the efficacy of eluxadoline for Irritable Bowel Syndrome compared with the placebo group. There are 2929 in the intervention group (eluxadoline) and 2934 in the placebo group. The primary outcome assessed was the odds ratio of efficacy of the drug eluxadoline vs placebo for the treatment of IBS. The odds of efficacy of the drug was significantly higher than the placebo group. ( OR = 1.68 [1.40; 2.02],95%-CI; P=0.0619, I^2=50.0%.) The mean change in quality of life was assessed through improvement in IBS-QOL. (MDS =1.97[-0.18;4.13],95%-CI ; p=0.095, I^2=52.9%.)
Discussion: The study conducted highlights the efficacy of Eluxadoline therapy in the management of IBS-Diarrhoea. Improved outcomes of the primary end-point efficacy and improvement of IBS-QOL score warrant the use of Eluxadoline as a therapeutic option in management of IBS - D.
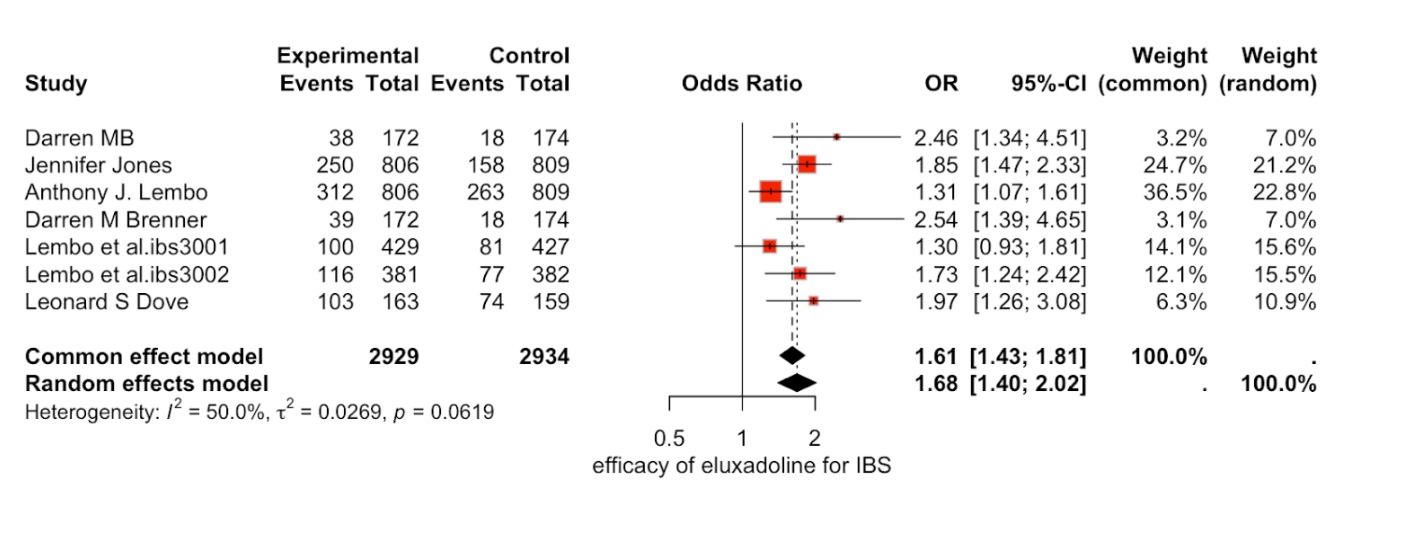
Figure: efficacy of eluxadoline for IBS
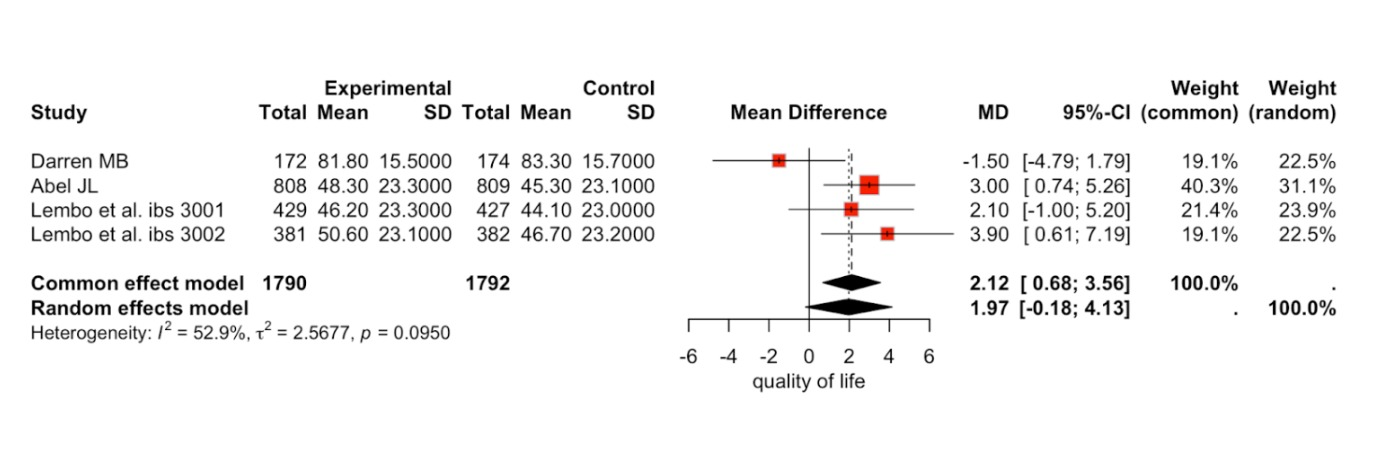
Figure: quality of life
Disclosures:
Shradha Chervittara Karaveetil indicated no relevant financial relationships.
Vinay Chandramouli Bellur indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Omar Oudit indicated no relevant financial relationships.
Allama Prabhu N S indicated no relevant financial relationships.
Rishikesh R. Magaji indicated no relevant financial relationships.
Trisha Chandra Mohan indicated no relevant financial relationships.
Aashray Alva indicated no relevant financial relationships.
Keerthi Balaji Babu Naidu indicated no relevant financial relationships.
Aryan Gupta indicated no relevant financial relationships.
Pavan Kumara Kasam Shiva indicated no relevant financial relationships.
Deepika A indicated no relevant financial relationships.
A. Shree Charan indicated no relevant financial relationships.
Vibhav MS indicated no relevant financial relationships.
Druvadeep Srinivas indicated no relevant financial relationships.
Vardhini Ganesh Iyer indicated no relevant financial relationships.
Sravani Bhavanam indicated no relevant financial relationships.
Shradha Chervittara Karaveetil, 1, Vinay Chandramouli Bellur, 2, Ananya Prasad, 1, Omar Oudit, DO3, Allama Prabhu N S, 4, Rishikesh R. Magaji, 5, Trisha Chandra Mohan, 5, Aashray Alva, 1, Keerthi Balaji Babu Naidu, 2, Aryan Gupta, 6, Pavan Kumara Kasam Shiva, 6, Deepika A, 6, A. Shree Charan, 7, Vibhav MS, 6, Druvadeep Srinivas, 8, Vardhini Ganesh Iyer, 5, Sravani Bhavanam, 9. P4909 - Safety and Efficacy of Eluxadoline for Irritable Bowel Syndrome (Diarrhea Predominant): Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1ramaiah medical college, Bangalore, Karnataka, India; 2Ramaiah medical college, Bangalore, Karnataka, India; 3Brookdale University Hospital Medical Center, Brooklyn, NY; 4Bangalore Medical College and Research Institute, Bangalore, Karnataka, India; 5BGS Global Institute of Medical Sciences, Bangalore, Karnataka, India; 6bangalore medical college and research institute, Bangalore, Karnataka, India; 7Ramaiah Medical College, Bangalore, Karnataka, India; 8rajarajeshwari medical college & hospital, Bangalore, Karnataka, India; 9Brookdale University Hospital Medical Center, Bangalore, Karnataka, India
Introduction: Irritable Bowel Syndrome with Diarrhea (IBS-D) is a chronic functional disorder marked by frequent loose stools, urgency, and abdominal discomfort, significantly impairing quality of life. Eluxadoline, a mixed μ- and κ-opioid receptor agonist and δ-opioid antagonist, has emerged as a targeted treatment for IBS-D. This meta-analysis evaluates the efficacy and safety of Eluxadoline in reducing diarrheal symptoms and improving overall symptom control in IBS-D patients.
Methods: The review conducted follows the PRISMA guidelines and major medical databases, which include PUBMED, Google Scholar and Science-Direct, were extensively searched using a comprehensive search term to identify and retrieve available articles. The articles included the assessment of outcomes following Eluxadoline therapy for Irritable bowel syndrome.
The data was analysed using the Meta, Metadata and the Metafor packages of R Studio. The Efficacy of Eluxadoline therapy was assessed by estimating the Odds ratio of the primary end point efficacy between the two groups. The QOL was assessed through the mean change in IBS-Quality of Life score. The Mantel-Haenszel method and the Inverse variance method were utilised to analyse the odds ratio and risk ratio. The I^2 test was used to assess the heterogeneity of the studies.
Results: The review includes a total of 7 articles that assessed the efficacy of eluxadoline for Irritable Bowel Syndrome compared with the placebo group. There are 2929 in the intervention group (eluxadoline) and 2934 in the placebo group. The primary outcome assessed was the odds ratio of efficacy of the drug eluxadoline vs placebo for the treatment of IBS. The odds of efficacy of the drug was significantly higher than the placebo group. ( OR = 1.68 [1.40; 2.02],95%-CI; P=0.0619, I^2=50.0%.) The mean change in quality of life was assessed through improvement in IBS-QOL. (MDS =1.97[-0.18;4.13],95%-CI ; p=0.095, I^2=52.9%.)
Discussion: The study conducted highlights the efficacy of Eluxadoline therapy in the management of IBS-Diarrhoea. Improved outcomes of the primary end-point efficacy and improvement of IBS-QOL score warrant the use of Eluxadoline as a therapeutic option in management of IBS - D.

Figure: efficacy of eluxadoline for IBS

Figure: quality of life
Disclosures:
Shradha Chervittara Karaveetil indicated no relevant financial relationships.
Vinay Chandramouli Bellur indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Omar Oudit indicated no relevant financial relationships.
Allama Prabhu N S indicated no relevant financial relationships.
Rishikesh R. Magaji indicated no relevant financial relationships.
Trisha Chandra Mohan indicated no relevant financial relationships.
Aashray Alva indicated no relevant financial relationships.
Keerthi Balaji Babu Naidu indicated no relevant financial relationships.
Aryan Gupta indicated no relevant financial relationships.
Pavan Kumara Kasam Shiva indicated no relevant financial relationships.
Deepika A indicated no relevant financial relationships.
A. Shree Charan indicated no relevant financial relationships.
Vibhav MS indicated no relevant financial relationships.
Druvadeep Srinivas indicated no relevant financial relationships.
Vardhini Ganesh Iyer indicated no relevant financial relationships.
Sravani Bhavanam indicated no relevant financial relationships.
Shradha Chervittara Karaveetil, 1, Vinay Chandramouli Bellur, 2, Ananya Prasad, 1, Omar Oudit, DO3, Allama Prabhu N S, 4, Rishikesh R. Magaji, 5, Trisha Chandra Mohan, 5, Aashray Alva, 1, Keerthi Balaji Babu Naidu, 2, Aryan Gupta, 6, Pavan Kumara Kasam Shiva, 6, Deepika A, 6, A. Shree Charan, 7, Vibhav MS, 6, Druvadeep Srinivas, 8, Vardhini Ganesh Iyer, 5, Sravani Bhavanam, 9. P4909 - Safety and Efficacy of Eluxadoline for Irritable Bowel Syndrome (Diarrhea Predominant): Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
