Tuesday Poster Session
Category: Esophagus
P4902 - Progression of Barrett’s Esophagus and Dysplasia Diagnosed by Wide-Area Transepithelial Sampling With 3-Dimensional Computer-Assisted Analysis (WATS3D): Results of Long-Term Follow-Up
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Natalie J. Wilson, MD (she/her/hers)
University of Minnesota
Chapel Hill, NC
Presenting Author(s)
Natalie J. Wilson, MD1, Michael S.. Smith, MD, MBA2, Matthew J. McKinley, FACG3, Arvind Trindade, MD4, William J.. Salyers, MD, MPH5, Vivek Kaul, MD, FACG6, Robert D. Odze, MD7, Nicholas J. Shaheen, MD, MPH, MACG8
1University of Minnesota, Minneapolis, MN; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3NYU Langone Health, Bethpage, NY; 4Rutgers Robert Wood Johnson University Hospital, New Brunswick, NJ; 5University of Kansas School of Medicine - Wichita, Wichita, KS; 6University of Rochester Medical Center, Rochester, NY; 7Tufts University, Boston, MA; 8University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: WATS3D, used as an adjunct to forceps biopsy (FB) for Barrett’s esophagus (BE) sampling, increases the yield of dysplasia and intestinal metaplasia (IM). However, the risk of neoplastic progression in WATS3D-diagnosed BE is poorly understood.
We aimed to evaluate the risk of progression to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) in the largest cohort to date of WATS3D-diagnosed columnar-lined esophagus without IM (non-IM CLE), nondysplastic BE (NDBE), indefinite (IND)/crypt dysplasia (CD), and low-grade dysplasia (LGD).
Methods: This was a retrospective study of patients undergoing WATS3D from 2013-2024. Data were collected from the WATS3D commercial database (CDx Diagnostics, Suffern, NY). Patients with ≥1 cm CLE and at least one follow-up endoscopy ≥12 months from index WATS3D were included. Patients with prior dysplasia or endoscopic therapy for BE were excluded.
The primary outcome was the rate of progression to HGD/EAC (detected by either WATS3D or FB) stratified by baseline WATS3D histology. Progression rate was reported as percentage/patient-year for each baseline histologic category.
Results: Of 337,206 patients undergoing WATS3D, 30,782 met inclusion criteria (Table 1). Overall, 76 progressed to HGD/EAC over a mean of 3.35 years (range, 1.0-17.92). Progression was detected in 37 patients by FB and 66 by WATS3D. The progression rate to HGD/EAC, as detected by FB, was 0.19%/pt-yr in NDBE, 1.0% in IND/CD, and 3.41% in LGD (Table 2). In comparison, the progression rate as detected by WATS3D was 0.10%, 0.32%, and 1.86% in NDBE, IND/CD, and LGD, respectively. Differences in progression rates as detected by WATS3D and FB were statistically significant in those with baseline NDBE (p=0.004) and baseline IND/CD (p=0.02) but not in those with LGD (p=0.43) or non-IM CLE (p=0.16). Increasing age, male sex, white race, and longer BE segment were more common in progressors vs. nonprogressors (Table 1).
Discussion: In this largest reported cohort to date, patients with LGD detected by WATS3D progressed to BE/EAC at similar rates whether detected by FB or WATS3D, with rates comparable to those previously reported for FB-diagnosed LGD. Progression rates were somewhat lower in WATS3D-diagnosed NDBE and IND/CD when the outcome was measured by WATS3D compared to FB. Progression rates in non-IM CLE were miniscule. These data suggest that LGD diagnosed by WATS3D should trigger the same treatment as that recommended for FB‑detected LGD.
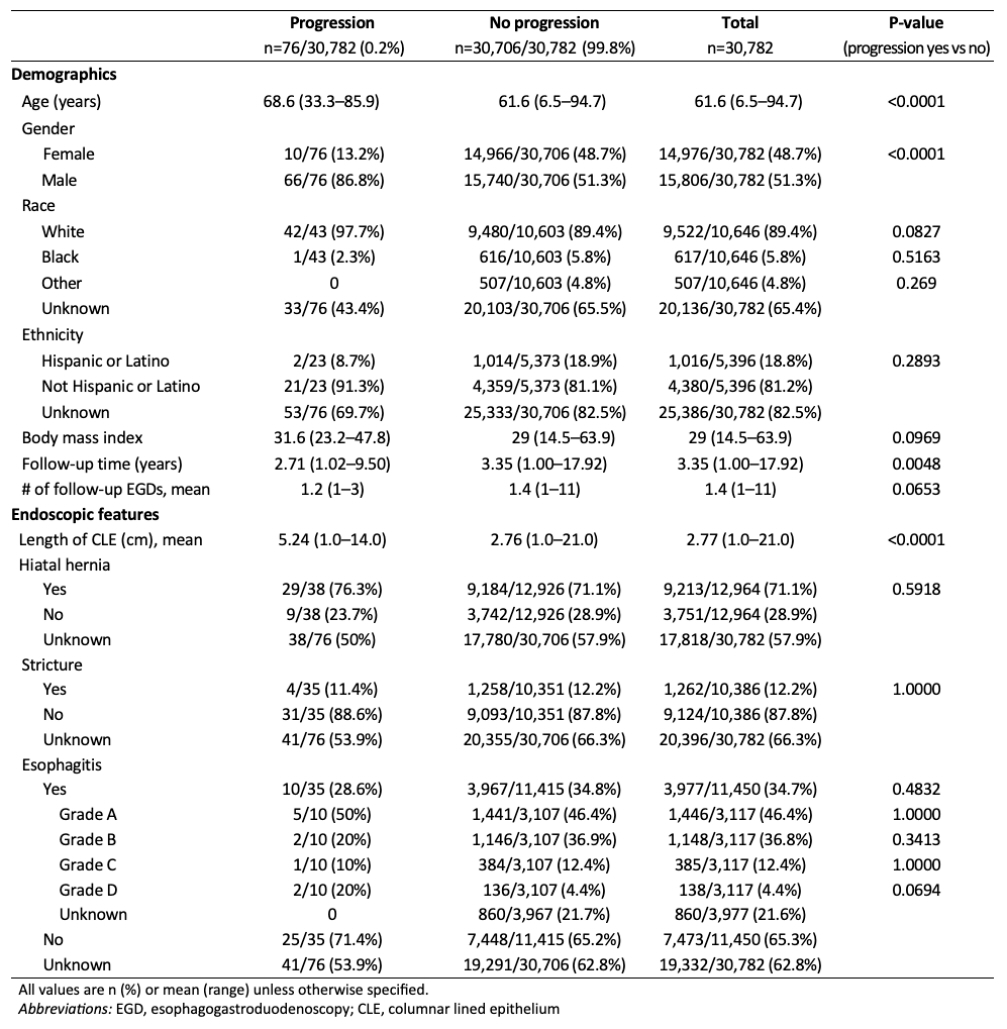
Figure: Table 1. Demographics and baseline endoscopic features, stratified by progression to high-grade dysplasia/esophageal adenocarcinoma detected by WATS3D
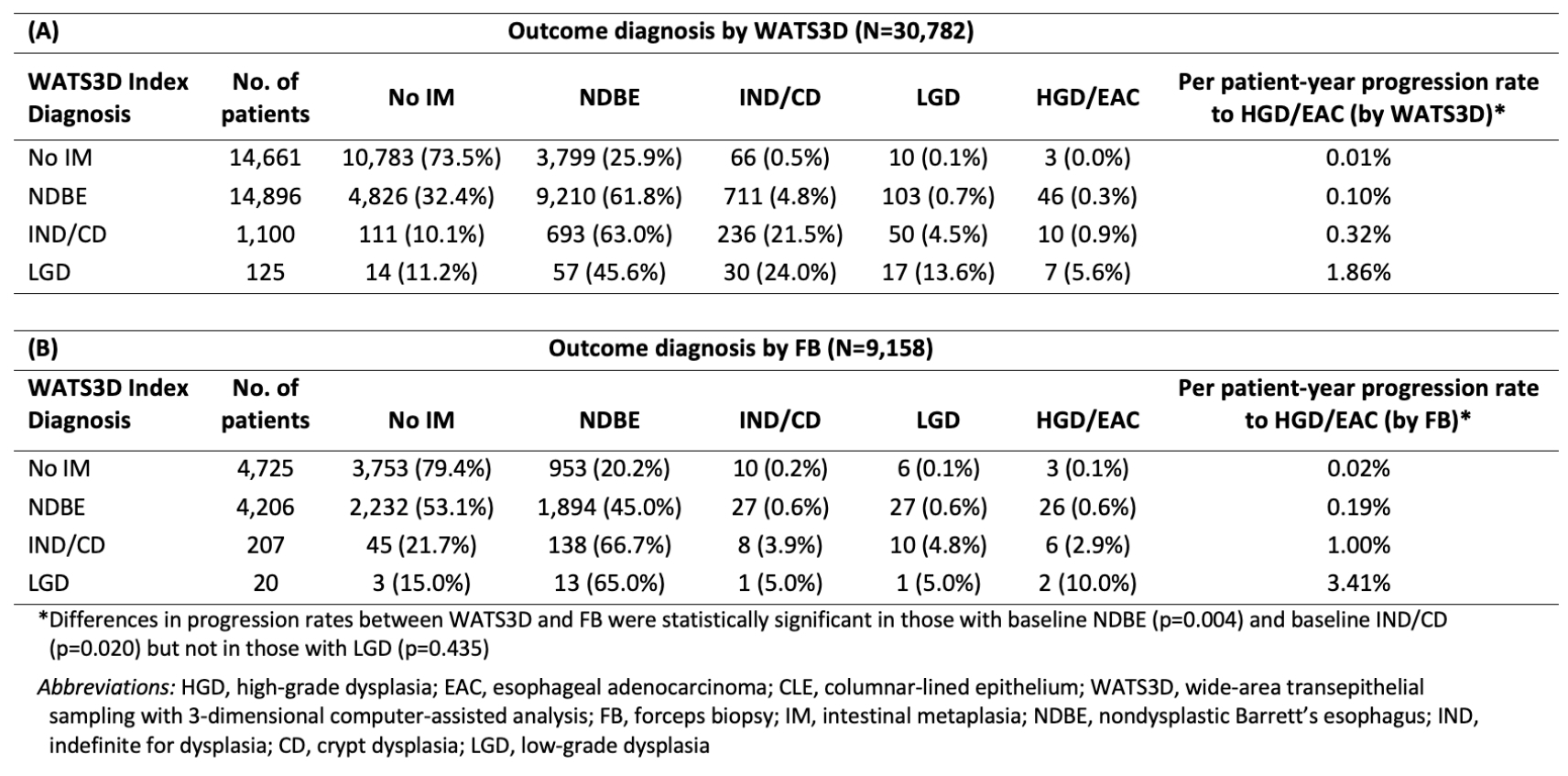
Figure: Table 2. Progression rates to HGD/EAC in patients with ≥1 cm CLE based on index WATS to outcome WATS (A) and index WATS to outcome FB (B)
Disclosures:
Natalie Wilson indicated no relevant financial relationships.
Michael Smith: Castle Biosciences – Consultant. CDx Diagnostics – Consultant. Lucid Diagnostics – Consultant. Provation Software – Consultant. Sanofi – Consultant. Steris Endoscopy – Consultant.
Matthew McKinley indicated no relevant financial relationships.
Arvind Trindade: Exact Sciences – Consultant, Grant/Research Support. Lucid Diagnostics – Consultant, Grant/Research Support.
William Salyers indicated no relevant financial relationships.
Vivek Kaul indicated no relevant financial relationships.
Robert Odze: CDx Medical – Consultant.
Nicholas Shaheen: Aqua – Grant/Research Support. CDx – Grant/Research Support. Cook Medical – Consultant. GIE Medical – Grant/Research Support. Interpace Diagnostics – Grant/Research Support. Lucid Diagnostics – Grant/Research Support. Medtronic – Grant/Research Support. Pentax – Grant/Research Support. Steris – Grant/Research Support.
Natalie J. Wilson, MD1, Michael S.. Smith, MD, MBA2, Matthew J. McKinley, FACG3, Arvind Trindade, MD4, William J.. Salyers, MD, MPH5, Vivek Kaul, MD, FACG6, Robert D. Odze, MD7, Nicholas J. Shaheen, MD, MPH, MACG8. P4902 - Progression of Barrett’s Esophagus and Dysplasia Diagnosed by Wide-Area Transepithelial Sampling With 3-Dimensional Computer-Assisted Analysis (WATS3D): Results of Long-Term Follow-Up, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Minnesota, Minneapolis, MN; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3NYU Langone Health, Bethpage, NY; 4Rutgers Robert Wood Johnson University Hospital, New Brunswick, NJ; 5University of Kansas School of Medicine - Wichita, Wichita, KS; 6University of Rochester Medical Center, Rochester, NY; 7Tufts University, Boston, MA; 8University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: WATS3D, used as an adjunct to forceps biopsy (FB) for Barrett’s esophagus (BE) sampling, increases the yield of dysplasia and intestinal metaplasia (IM). However, the risk of neoplastic progression in WATS3D-diagnosed BE is poorly understood.
We aimed to evaluate the risk of progression to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) in the largest cohort to date of WATS3D-diagnosed columnar-lined esophagus without IM (non-IM CLE), nondysplastic BE (NDBE), indefinite (IND)/crypt dysplasia (CD), and low-grade dysplasia (LGD).
Methods: This was a retrospective study of patients undergoing WATS3D from 2013-2024. Data were collected from the WATS3D commercial database (CDx Diagnostics, Suffern, NY). Patients with ≥1 cm CLE and at least one follow-up endoscopy ≥12 months from index WATS3D were included. Patients with prior dysplasia or endoscopic therapy for BE were excluded.
The primary outcome was the rate of progression to HGD/EAC (detected by either WATS3D or FB) stratified by baseline WATS3D histology. Progression rate was reported as percentage/patient-year for each baseline histologic category.
Results: Of 337,206 patients undergoing WATS3D, 30,782 met inclusion criteria (Table 1). Overall, 76 progressed to HGD/EAC over a mean of 3.35 years (range, 1.0-17.92). Progression was detected in 37 patients by FB and 66 by WATS3D. The progression rate to HGD/EAC, as detected by FB, was 0.19%/pt-yr in NDBE, 1.0% in IND/CD, and 3.41% in LGD (Table 2). In comparison, the progression rate as detected by WATS3D was 0.10%, 0.32%, and 1.86% in NDBE, IND/CD, and LGD, respectively. Differences in progression rates as detected by WATS3D and FB were statistically significant in those with baseline NDBE (p=0.004) and baseline IND/CD (p=0.02) but not in those with LGD (p=0.43) or non-IM CLE (p=0.16). Increasing age, male sex, white race, and longer BE segment were more common in progressors vs. nonprogressors (Table 1).
Discussion: In this largest reported cohort to date, patients with LGD detected by WATS3D progressed to BE/EAC at similar rates whether detected by FB or WATS3D, with rates comparable to those previously reported for FB-diagnosed LGD. Progression rates were somewhat lower in WATS3D-diagnosed NDBE and IND/CD when the outcome was measured by WATS3D compared to FB. Progression rates in non-IM CLE were miniscule. These data suggest that LGD diagnosed by WATS3D should trigger the same treatment as that recommended for FB‑detected LGD.

Figure: Table 1. Demographics and baseline endoscopic features, stratified by progression to high-grade dysplasia/esophageal adenocarcinoma detected by WATS3D

Figure: Table 2. Progression rates to HGD/EAC in patients with ≥1 cm CLE based on index WATS to outcome WATS (A) and index WATS to outcome FB (B)
Disclosures:
Natalie Wilson indicated no relevant financial relationships.
Michael Smith: Castle Biosciences – Consultant. CDx Diagnostics – Consultant. Lucid Diagnostics – Consultant. Provation Software – Consultant. Sanofi – Consultant. Steris Endoscopy – Consultant.
Matthew McKinley indicated no relevant financial relationships.
Arvind Trindade: Exact Sciences – Consultant, Grant/Research Support. Lucid Diagnostics – Consultant, Grant/Research Support.
William Salyers indicated no relevant financial relationships.
Vivek Kaul indicated no relevant financial relationships.
Robert Odze: CDx Medical – Consultant.
Nicholas Shaheen: Aqua – Grant/Research Support. CDx – Grant/Research Support. Cook Medical – Consultant. GIE Medical – Grant/Research Support. Interpace Diagnostics – Grant/Research Support. Lucid Diagnostics – Grant/Research Support. Medtronic – Grant/Research Support. Pentax – Grant/Research Support. Steris – Grant/Research Support.
Natalie J. Wilson, MD1, Michael S.. Smith, MD, MBA2, Matthew J. McKinley, FACG3, Arvind Trindade, MD4, William J.. Salyers, MD, MPH5, Vivek Kaul, MD, FACG6, Robert D. Odze, MD7, Nicholas J. Shaheen, MD, MPH, MACG8. P4902 - Progression of Barrett’s Esophagus and Dysplasia Diagnosed by Wide-Area Transepithelial Sampling With 3-Dimensional Computer-Assisted Analysis (WATS3D): Results of Long-Term Follow-Up, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
