Tuesday Poster Session
Category: Esophagus
P4899 - Minimally Invasive Biomarker Response to Treatment in Eosinophilic Esophagitis: A Systematic Review and Pooled Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- HN
Hao The Nguyen
Rocky Vista University
waldorf, MD
Presenting Author(s)
Hao The Nguyen, 1, Arman Vaghefi, DO1, Paul Pardo, 2, Shania Darville, 3, Michelle Hor, MD4
1Rocky Vista University, Colorado Springs, CO; 2University of South Florida, Clearwater, FL; 3University of South Florida, Tampa, FL; 4University of Colorado School of Medicine, Colorado Springs, CO
Introduction: Non-invasive biomarkers may help monitor treatment response in EoE and reduce reliance on repeated endoscopy, but their clinical utility remains unclear. This study evaluates post-treatment changes in serum biomarkers to identify consistent indicators of therapeutic response in adults with EoE.
Methods: This sub-analysis derives from a PROSPERO-registered systematic review (CRD420251040649). A systematic search of PubMed, Embase, and Cochrane Central (Jan 2014–May 2025) was conducted independently by two reviewers. Studies were included if they reported paired pre- and post-treatment biomarker values in biopsy-confirmed adult EoE. Disagreements were resolved by a third reviewer. Extracted biomarkers included absolute eosinophil count (AEC), eosinophilic cationic protein (ECP), eosinophil-derived neurotoxin (EDN), major basic protein (MBP), eotaxin-3, IL-5, and IL-13. Data included biomarker values, treatment type, and response stratification. Pooled mean changes (Δ) were calculated using fixed-effect, sample-size weighting. Standard deviations (SD) were pooled using the standard variance formula; when missing, SDs were estimated using the Wan et al. (2014) method with r = .50. All estimates include 95% confidence intervals (CI).
Results: 10 of 854 screened studies met criteria, contributing ~300 biomarker data points from 177 unique adults. Treatments included steroids, PPIs, and dietary elimination, though stratification was inconsistent. AEC decreased significantly from 0.324×10⁹/L to 0.118×10⁹/L (Δ = –0.173, 95% CI –0.25 to –0.09; p = .001). ECP (Δ = –24.9 ng/mL; CI –36.7 to –13.0; p ≤ .006) and EDN (Δ = –54.4 ng/mL; CI –83.3 to –25.6; p = .002) also significantly decreased. MBP, IL-5, and IL-13 showed inconsistent changes that were not significant. SDs were imputed in 4 studies, introducing some imprecision but limiting loss of statistical power. Because each pooled estimate included ≤ 3 methodologically similar studies and a random-effects sensitivity test did not shift the point estimates, a fixed-effect model was retained.
Discussion: Significant post-treatment declines in AEC, ECP and EDN across pooled studies highlights their potential as objective serum indicators of therapeutic response in adult EoE, warranting prospective validation. MBP and chemokine markers showed non-significant, heterogeneous changes. Standardized reporting and response stratification in future studies remain critical for clinical translation.
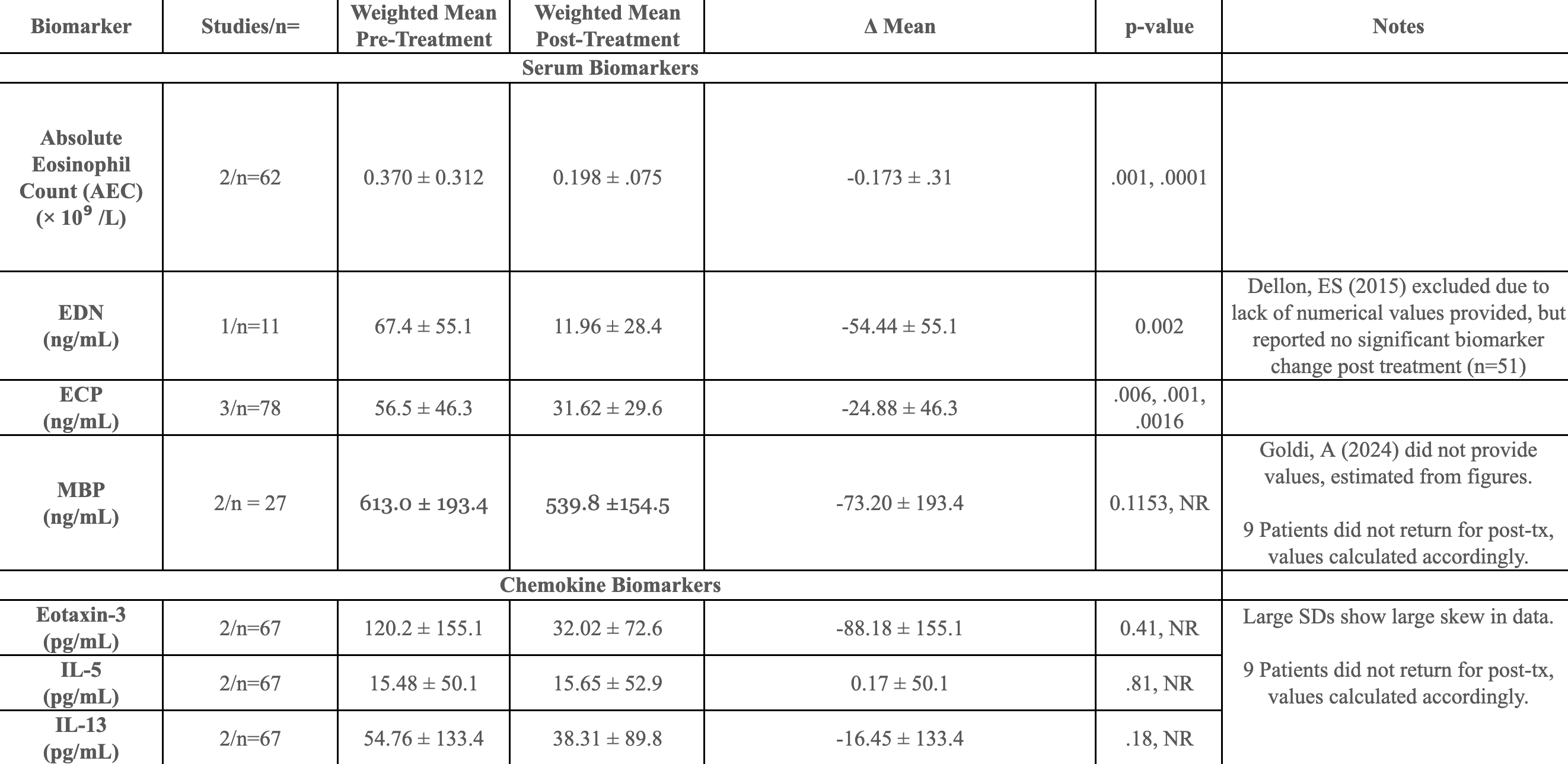
Figure: Weighted mean changes in eosinophil-related serum and chemokine biomarkers pre- and post-treatment, with associated statistical significance across included studies.
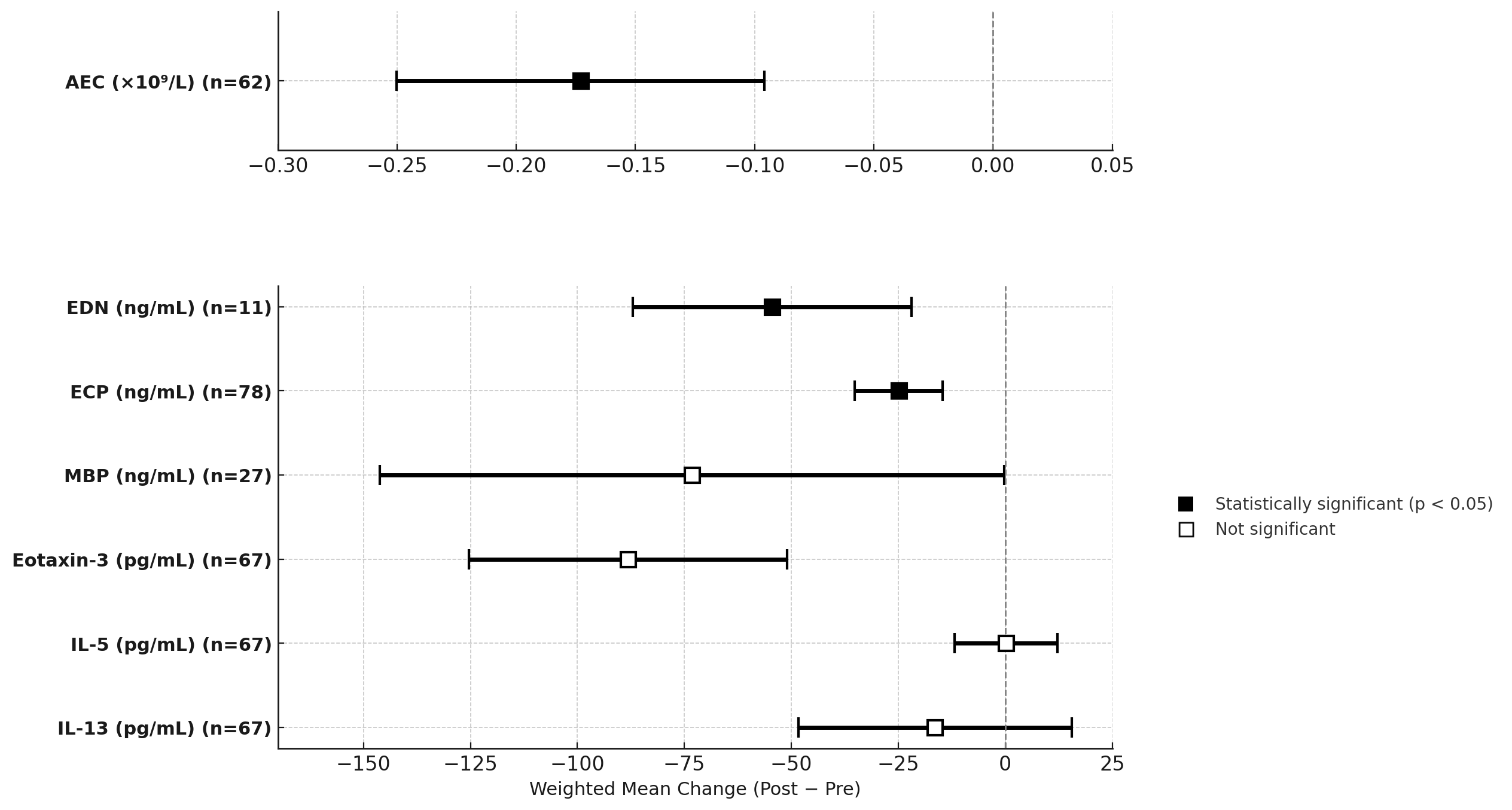
Figure: Forest plot showing weighted mean change and 95% confidence intervals for the biomarkers post-treatment. Statistically significant reductions observed in AEC, EDN, and ECP (filled squares); other changes were not significant (open squares).
Disclosures:
Hao The Nguyen indicated no relevant financial relationships.
Arman Vaghefi indicated no relevant financial relationships.
Paul Pardo indicated no relevant financial relationships.
Shania Darville indicated no relevant financial relationships.
Michelle Hor indicated no relevant financial relationships.
Hao The Nguyen, 1, Arman Vaghefi, DO1, Paul Pardo, 2, Shania Darville, 3, Michelle Hor, MD4. P4899 - Minimally Invasive Biomarker Response to Treatment in Eosinophilic Esophagitis: A Systematic Review and Pooled Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rocky Vista University, Colorado Springs, CO; 2University of South Florida, Clearwater, FL; 3University of South Florida, Tampa, FL; 4University of Colorado School of Medicine, Colorado Springs, CO
Introduction: Non-invasive biomarkers may help monitor treatment response in EoE and reduce reliance on repeated endoscopy, but their clinical utility remains unclear. This study evaluates post-treatment changes in serum biomarkers to identify consistent indicators of therapeutic response in adults with EoE.
Methods: This sub-analysis derives from a PROSPERO-registered systematic review (CRD420251040649). A systematic search of PubMed, Embase, and Cochrane Central (Jan 2014–May 2025) was conducted independently by two reviewers. Studies were included if they reported paired pre- and post-treatment biomarker values in biopsy-confirmed adult EoE. Disagreements were resolved by a third reviewer. Extracted biomarkers included absolute eosinophil count (AEC), eosinophilic cationic protein (ECP), eosinophil-derived neurotoxin (EDN), major basic protein (MBP), eotaxin-3, IL-5, and IL-13. Data included biomarker values, treatment type, and response stratification. Pooled mean changes (Δ) were calculated using fixed-effect, sample-size weighting. Standard deviations (SD) were pooled using the standard variance formula; when missing, SDs were estimated using the Wan et al. (2014) method with r = .50. All estimates include 95% confidence intervals (CI).
Results: 10 of 854 screened studies met criteria, contributing ~300 biomarker data points from 177 unique adults. Treatments included steroids, PPIs, and dietary elimination, though stratification was inconsistent. AEC decreased significantly from 0.324×10⁹/L to 0.118×10⁹/L (Δ = –0.173, 95% CI –0.25 to –0.09; p = .001). ECP (Δ = –24.9 ng/mL; CI –36.7 to –13.0; p ≤ .006) and EDN (Δ = –54.4 ng/mL; CI –83.3 to –25.6; p = .002) also significantly decreased. MBP, IL-5, and IL-13 showed inconsistent changes that were not significant. SDs were imputed in 4 studies, introducing some imprecision but limiting loss of statistical power. Because each pooled estimate included ≤ 3 methodologically similar studies and a random-effects sensitivity test did not shift the point estimates, a fixed-effect model was retained.
Discussion: Significant post-treatment declines in AEC, ECP and EDN across pooled studies highlights their potential as objective serum indicators of therapeutic response in adult EoE, warranting prospective validation. MBP and chemokine markers showed non-significant, heterogeneous changes. Standardized reporting and response stratification in future studies remain critical for clinical translation.

Figure: Weighted mean changes in eosinophil-related serum and chemokine biomarkers pre- and post-treatment, with associated statistical significance across included studies.

Figure: Forest plot showing weighted mean change and 95% confidence intervals for the biomarkers post-treatment. Statistically significant reductions observed in AEC, EDN, and ECP (filled squares); other changes were not significant (open squares).
Disclosures:
Hao The Nguyen indicated no relevant financial relationships.
Arman Vaghefi indicated no relevant financial relationships.
Paul Pardo indicated no relevant financial relationships.
Shania Darville indicated no relevant financial relationships.
Michelle Hor indicated no relevant financial relationships.
Hao The Nguyen, 1, Arman Vaghefi, DO1, Paul Pardo, 2, Shania Darville, 3, Michelle Hor, MD4. P4899 - Minimally Invasive Biomarker Response to Treatment in Eosinophilic Esophagitis: A Systematic Review and Pooled Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
