Tuesday Poster Session
Category: Diet, Nutrition, and Obesity
P4826 - Reduced Risk of Iron Deficiency Anemia With GLP-1 Use in Celiac Disease Patients
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jonathan Ghobrial, MD
Allegheny Health Network Medicine Institute
Pittsburgh, PA
Presenting Author(s)
Jonathan Ghobrial, MD1, Mirella Youssef, MS2, Ahmed Abdelwahab, MD3, Jonathan Ragheb, MD4
1Allegheny Health Network Medicine Institute, Pittsburgh, PA; 2The Ohio State University College of Medicine, Pittsburgh, PA; 3University of Toledo College Medicine and Life Sciences, Toledo, OH; 4AdventHealth, Lake Mary, FL
Introduction: Celiac disease is an autoimmune condition characterized by chronic intestinal inflammation and nutrient malabsorption. GLP-1 receptor agonists, widely used in the management of type 2 diabetes and obesity, are known to influence gastrointestinal function and nutrient uptake. Their impact on nutritional deficiencies and clinical outcomes in patients with celiac disease has not been well characterized. This study is the first to evaluate the association between GLP-1 agonist use and key clinical outcomes, including anemia, transfusion, hospitalization, and mortality, in a large-scale analysis of patients with celiac disease.
Methods: Data were sourced from TriNetX, a federated de-identified health research network including 70 healthcare organizations across the U.S. Patients with celiac disease (K90.0) were identified using ICD-10 codes and stratified according to documented exposure to GLP-1 agonists (semaglutide, dulaglutide, liraglutide, or exenatide). Propensity score matching (1:1) was performed on demographics, comorbidities, and relevant lab values. Clinical outcomes included mortality, iron deficiency anemia (IDA), transfusion, B12/folate deficiency, GI bleeding, and hemoglobin trends. Risk analysis and Kaplan-Meier survival estimates were used to assess outcome differences, with results reported using risk ratios, hazard ratios, and 95% confidence intervals.
Results: A total of 18,582 matched patients (9,291 per group) were analyzed. GLP-1 users had significantly lower mortality (1.9% vs. 3.3%, HR=1.82, 95% CI: 1.51–2.20, p< 0.001) and fewer transfusions (0.9% vs. 1.3%, HR=1.50, p=0.004). Non-GLP1 users had a higher risk of iron deficiency anemia (5.4% vs. 6.5%, HR=1.26, p=0.001). Risk of B12 or folate deficiency was also reduced in the GLP-1 group (0.8% vs. 1.3%, HR=1.62, p=0.001). GI bleeding rates were slightly higher in the GLP-1 group by time-to-event analysis (HR=1.21, p=0.044), though absolute risk was similar.
Discussion: GLP-1 use conferred a significant survival benefit as well as reduced risk of iron deficiency anemia in celiac patients. In a patient population that is prone to nutritional deficiencies due to malabsorption, particularly IDA, these results highlight a potential way to reduce these deficiencies. Although by what means GLP-1 agonists confer a reduced risk of IDA in celiac patients is not understood from this study, further studies are warranted to further evaluate these findings.
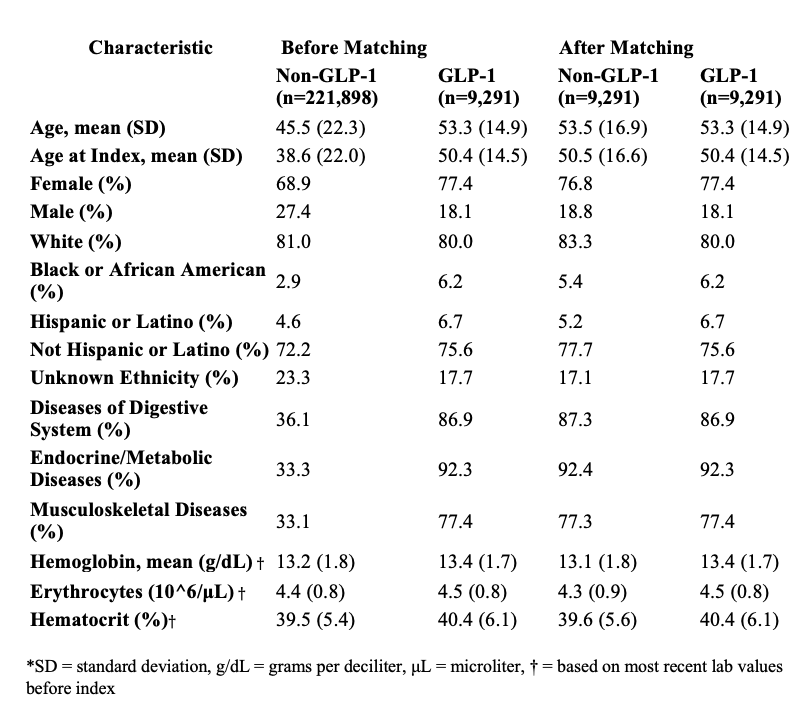
Figure: Figure 1: Kaplan-Meier survival curves comparing GLP-1 and non-GLP-1 users among celiac disease patients for six outcomes. Hazard ratios, 95% confidence intervals, and log-rank p-values are shown for each outcome.
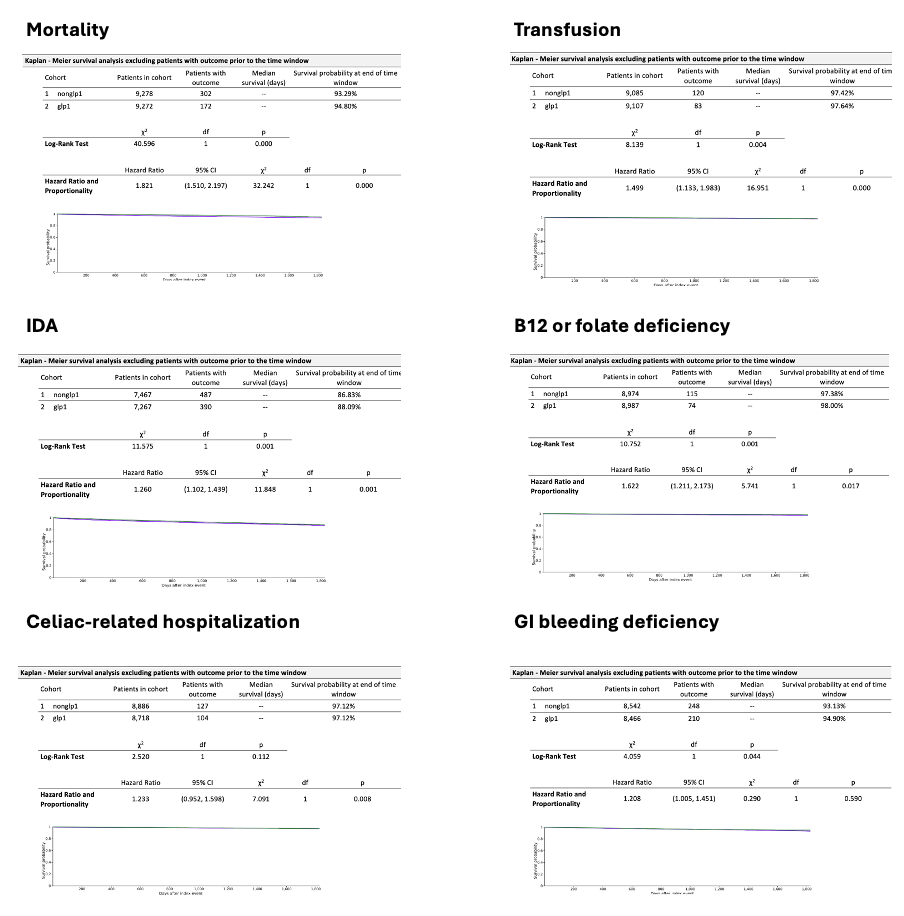
Figure: Table 1: Characteristics of patients with celiac disease with both cohorts of non-GLP-1 and GLP-1 exposure before and after propensity score matching.
Disclosures:
Jonathan Ghobrial indicated no relevant financial relationships.
Mirella Youssef indicated no relevant financial relationships.
Ahmed Abdelwahab indicated no relevant financial relationships.
Jonathan Ragheb indicated no relevant financial relationships.
Jonathan Ghobrial, MD1, Mirella Youssef, MS2, Ahmed Abdelwahab, MD3, Jonathan Ragheb, MD4. P4826 - Reduced Risk of Iron Deficiency Anemia With GLP-1 Use in Celiac Disease Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny Health Network Medicine Institute, Pittsburgh, PA; 2The Ohio State University College of Medicine, Pittsburgh, PA; 3University of Toledo College Medicine and Life Sciences, Toledo, OH; 4AdventHealth, Lake Mary, FL
Introduction: Celiac disease is an autoimmune condition characterized by chronic intestinal inflammation and nutrient malabsorption. GLP-1 receptor agonists, widely used in the management of type 2 diabetes and obesity, are known to influence gastrointestinal function and nutrient uptake. Their impact on nutritional deficiencies and clinical outcomes in patients with celiac disease has not been well characterized. This study is the first to evaluate the association between GLP-1 agonist use and key clinical outcomes, including anemia, transfusion, hospitalization, and mortality, in a large-scale analysis of patients with celiac disease.
Methods: Data were sourced from TriNetX, a federated de-identified health research network including 70 healthcare organizations across the U.S. Patients with celiac disease (K90.0) were identified using ICD-10 codes and stratified according to documented exposure to GLP-1 agonists (semaglutide, dulaglutide, liraglutide, or exenatide). Propensity score matching (1:1) was performed on demographics, comorbidities, and relevant lab values. Clinical outcomes included mortality, iron deficiency anemia (IDA), transfusion, B12/folate deficiency, GI bleeding, and hemoglobin trends. Risk analysis and Kaplan-Meier survival estimates were used to assess outcome differences, with results reported using risk ratios, hazard ratios, and 95% confidence intervals.
Results: A total of 18,582 matched patients (9,291 per group) were analyzed. GLP-1 users had significantly lower mortality (1.9% vs. 3.3%, HR=1.82, 95% CI: 1.51–2.20, p< 0.001) and fewer transfusions (0.9% vs. 1.3%, HR=1.50, p=0.004). Non-GLP1 users had a higher risk of iron deficiency anemia (5.4% vs. 6.5%, HR=1.26, p=0.001). Risk of B12 or folate deficiency was also reduced in the GLP-1 group (0.8% vs. 1.3%, HR=1.62, p=0.001). GI bleeding rates were slightly higher in the GLP-1 group by time-to-event analysis (HR=1.21, p=0.044), though absolute risk was similar.
Discussion: GLP-1 use conferred a significant survival benefit as well as reduced risk of iron deficiency anemia in celiac patients. In a patient population that is prone to nutritional deficiencies due to malabsorption, particularly IDA, these results highlight a potential way to reduce these deficiencies. Although by what means GLP-1 agonists confer a reduced risk of IDA in celiac patients is not understood from this study, further studies are warranted to further evaluate these findings.

Figure: Figure 1: Kaplan-Meier survival curves comparing GLP-1 and non-GLP-1 users among celiac disease patients for six outcomes. Hazard ratios, 95% confidence intervals, and log-rank p-values are shown for each outcome.

Figure: Table 1: Characteristics of patients with celiac disease with both cohorts of non-GLP-1 and GLP-1 exposure before and after propensity score matching.
Disclosures:
Jonathan Ghobrial indicated no relevant financial relationships.
Mirella Youssef indicated no relevant financial relationships.
Ahmed Abdelwahab indicated no relevant financial relationships.
Jonathan Ragheb indicated no relevant financial relationships.
Jonathan Ghobrial, MD1, Mirella Youssef, MS2, Ahmed Abdelwahab, MD3, Jonathan Ragheb, MD4. P4826 - Reduced Risk of Iron Deficiency Anemia With GLP-1 Use in Celiac Disease Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
