Tuesday Poster Session
Category: Colorectal Cancer Prevention
P4808 - FIT-based Colorectal Cancer Screening Completion Rates (Initial Test and Follow-up Colonoscopy) by Health Facility Type: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

A. Burak Ozbay, PhD
Exact Sciences
Madison, WI
Presenting Author(s)
Xiting Cao, PhD1, Vahab Vahdat, PhD1, Heather A. Johnson, MLIS1, Gina Thompson, PhD1, Joyce Kong, PharmD2, Irem Betül Koçak, PhD3, Carol Kirshner, MS4, A. Burak Ozbay, PhD1, Thomas Imperiale, MD5, Mohammad Dehghani, PhD6, Michael Dore, MD7, Portia Zaire, PhD8, A. Mark Fendrick, MD8, Paul J. Limburg, MD1
1Exact Sciences, Madison, WI; 2Rutgers University, Piscataway, NJ; 3Value Analytics Labs, Madison, WI; 4Value Analytics Labs, Boston, MA; 5Indiana University School of Medicine, Indianapolis, IN; 6Northeastern University, Boston, MA; 7Duke University School of Medicine, Durham, NC; 8University of Michigan, Ann Arbor, MI
Introduction: Stool-based strategies for colorectal cancer (CRC) screening require completion of the initial test and follow-up colonoscopy (FU-COL) when positive. Health facilities that employ the fecal immunochemical test (FIT) for CRC screening may leverage patient navigation and/or other support interventions to increase FIT and FU-COL adherence. The objective of this study was to assess the effect of these interventions on both FIT adherence and FU-COL adherence within a year at Federally Qualified Health Centers (FQHCs), Safety-Net Facilities (SNFs), Rural Health Clinics (RHCs), and Indian Health Service (IHS) facilities.
Methods: We searched only PubMed, EMBASE, and reference lists related to those articles to identify studies describing CRC screening test adherence. We included US-based, English-language manuscripts published from 1/1/2013 to 11/10/2023 and abstracts from 1/1/2020 to 11/10/2023. We excluded high-risk populations and studies describing population-based adherence. A random effects model was used to calculate the adherence rates for FIT and FU-COL after a positive FIT.
Results: From review of 5,761 unique titles/abstracts, we included 38 FIT and 9 FU-COL adherence studies. We identified 16 and 12 individual interventions for FIT and FU-COL, respectively, which were implemented in 405 and 40 combinations, respectively. The most common interventions for FIT were postal communication recommending screening (13.8%), provision of test instructions (13.6%), CRC education (12.6%), and mailed FIT (11.9%). The most common intervention for FU-COL was scheduling support (20%). Overall, FIT adherence was 35.7% (95% CI: 30.1-41.4) and was higher among those receiving any intervention compared to the usual care (39.4% vs. 26.1%, p=0.033). Overall FU-COL adherence after a positive FIT was 46.6% (95% CI: 41.1-52.1) and was numerically higher with intervention compared to usual care (52.0% vs. 45.3%, p=0.39). Table 1 shows adherence by facility type.
Discussion: Even with navigation and/or other support interventions, FIT adherence and FU-COL after a positive FIT are suboptimal. Unequal access to FU-COL may contribute to disparities across health system types, with low adherence in resource-limited settings. These findings should be informative to CRC screening program decision-makers when considering the balance of resource investment vs. achievable benefits from FIT-based strategies.
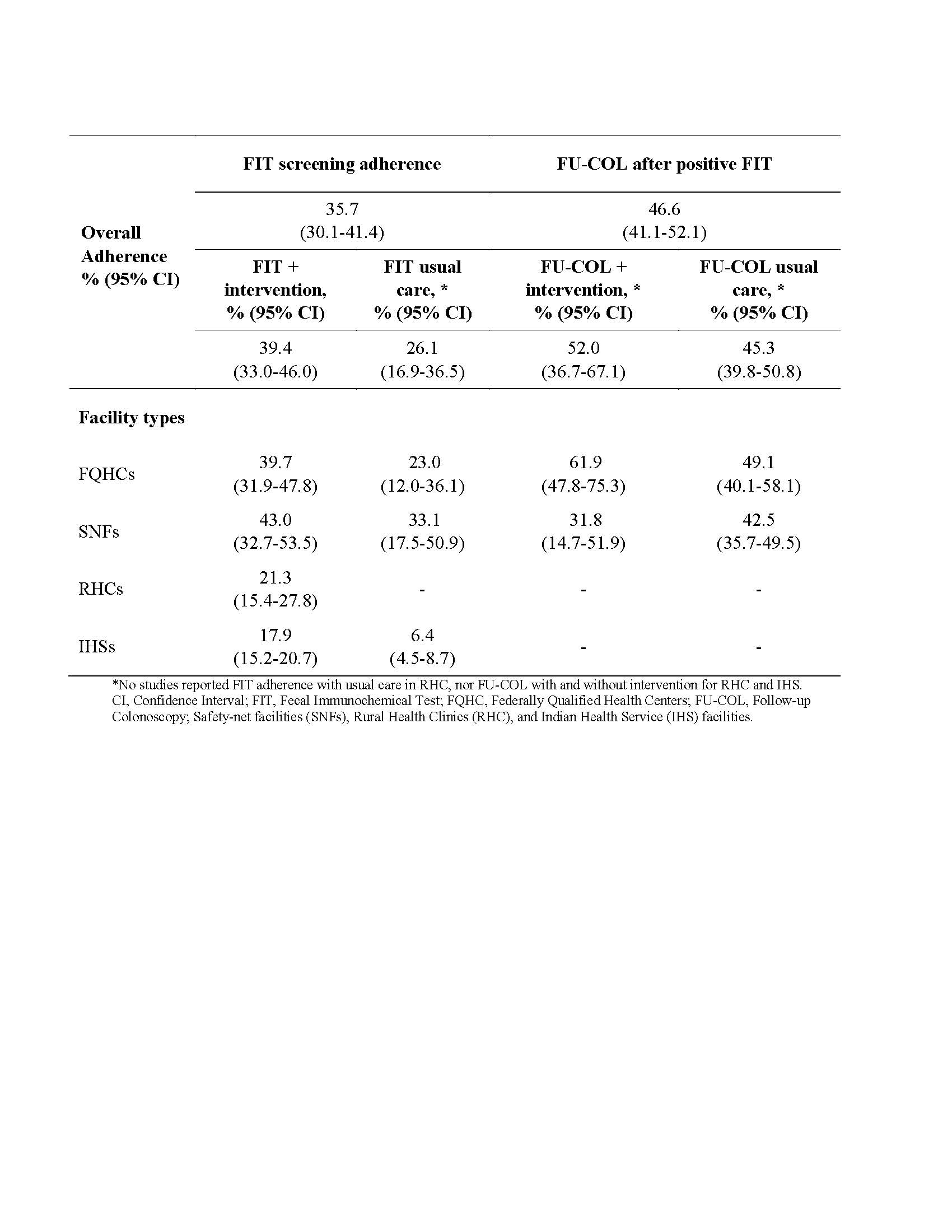
Figure: Table 1. FIT and follow-up colonoscopy adherence rates with and without intervention by facility type
Disclosures:
Xiting Cao: Exact Sciences – Employee.
Vahab Vahdat: Exact Sciences – Employee.
Heather A. Johnson: Exact Science – Employee.
Gina Thompson: Exact Sciences – Employee.
Joyce Kong indicated no relevant financial relationships.
Irem Betül Koçak: Exact Sciences – Independent Contractor.
Carol Kirshner: Exact Sciences – Independent Contractor.
A. Burak Ozbay: Exact Sciences – Employee.
Thomas Imperiale: Cook Medical – Consultant. Exact Sciences – Advisor or Review Panel Member, Consultant. Exact Sciences Corp – Grant/Research Support.
Mohammad Dehghani: Exact Sciences – Advisor or Review Panel Member, Consultant. Exact Sciences – Consultant.
Michael Dore indicated no relevant financial relationships.
Portia Zaire indicated no relevant financial relationships.
A. Mark Fendrick: AbbVie – Consultant. Agency for Healthcare Research and Quality – Grant/Research Support. Amgen – Consultant. Arnold Ventures – Grant/Research Support. Centers for Medicare and Medicaid Services. – Grant/Research Support. Centivo – Consultant. Community Oncology Association – Consultant. Covered California – Consultant. EmblemHealth – Consultant. Exact Sciences – Consultant. Freedman Health – Consultant. Gary and Mary West Health Policy Center – Grant/Research Support. GRAIL – Consultant. Harvard University – Consultant. Health & Wellness Innovations – Consultant. Health at Scale Technologies – Consultant. MedZed – Consultant. National Pharmaceutical Council – Grant/Research Support. Patient-Centered Outcomes Research Institute – Grant/Research Support. Penguin Pay – Consultant. Pharmaceutical Research and Manufacturers of America – Grant/Research Support. Risalto – Consultant. Robert Wood Johnson Foundation – Grant/Research Support. Sempre Health – Consultant. State of Michigan – Grant/Research Support. State of Minnesota – Consultant. U.S. Department of Defense – Consultant. Virginia Center for Health Innovation – Consultant. Wellthy – Consultant. Zansors – Consultant.
Paul J. Limburg: Exact Sciences – Employee.
Xiting Cao, PhD1, Vahab Vahdat, PhD1, Heather A. Johnson, MLIS1, Gina Thompson, PhD1, Joyce Kong, PharmD2, Irem Betül Koçak, PhD3, Carol Kirshner, MS4, A. Burak Ozbay, PhD1, Thomas Imperiale, MD5, Mohammad Dehghani, PhD6, Michael Dore, MD7, Portia Zaire, PhD8, A. Mark Fendrick, MD8, Paul J. Limburg, MD1. P4808 - FIT-based Colorectal Cancer Screening Completion Rates (Initial Test and Follow-up Colonoscopy) by Health Facility Type: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Exact Sciences, Madison, WI; 2Rutgers University, Piscataway, NJ; 3Value Analytics Labs, Madison, WI; 4Value Analytics Labs, Boston, MA; 5Indiana University School of Medicine, Indianapolis, IN; 6Northeastern University, Boston, MA; 7Duke University School of Medicine, Durham, NC; 8University of Michigan, Ann Arbor, MI
Introduction: Stool-based strategies for colorectal cancer (CRC) screening require completion of the initial test and follow-up colonoscopy (FU-COL) when positive. Health facilities that employ the fecal immunochemical test (FIT) for CRC screening may leverage patient navigation and/or other support interventions to increase FIT and FU-COL adherence. The objective of this study was to assess the effect of these interventions on both FIT adherence and FU-COL adherence within a year at Federally Qualified Health Centers (FQHCs), Safety-Net Facilities (SNFs), Rural Health Clinics (RHCs), and Indian Health Service (IHS) facilities.
Methods: We searched only PubMed, EMBASE, and reference lists related to those articles to identify studies describing CRC screening test adherence. We included US-based, English-language manuscripts published from 1/1/2013 to 11/10/2023 and abstracts from 1/1/2020 to 11/10/2023. We excluded high-risk populations and studies describing population-based adherence. A random effects model was used to calculate the adherence rates for FIT and FU-COL after a positive FIT.
Results: From review of 5,761 unique titles/abstracts, we included 38 FIT and 9 FU-COL adherence studies. We identified 16 and 12 individual interventions for FIT and FU-COL, respectively, which were implemented in 405 and 40 combinations, respectively. The most common interventions for FIT were postal communication recommending screening (13.8%), provision of test instructions (13.6%), CRC education (12.6%), and mailed FIT (11.9%). The most common intervention for FU-COL was scheduling support (20%). Overall, FIT adherence was 35.7% (95% CI: 30.1-41.4) and was higher among those receiving any intervention compared to the usual care (39.4% vs. 26.1%, p=0.033). Overall FU-COL adherence after a positive FIT was 46.6% (95% CI: 41.1-52.1) and was numerically higher with intervention compared to usual care (52.0% vs. 45.3%, p=0.39). Table 1 shows adherence by facility type.
Discussion: Even with navigation and/or other support interventions, FIT adherence and FU-COL after a positive FIT are suboptimal. Unequal access to FU-COL may contribute to disparities across health system types, with low adherence in resource-limited settings. These findings should be informative to CRC screening program decision-makers when considering the balance of resource investment vs. achievable benefits from FIT-based strategies.

Figure: Table 1. FIT and follow-up colonoscopy adherence rates with and without intervention by facility type
Disclosures:
Xiting Cao: Exact Sciences – Employee.
Vahab Vahdat: Exact Sciences – Employee.
Heather A. Johnson: Exact Science – Employee.
Gina Thompson: Exact Sciences – Employee.
Joyce Kong indicated no relevant financial relationships.
Irem Betül Koçak: Exact Sciences – Independent Contractor.
Carol Kirshner: Exact Sciences – Independent Contractor.
A. Burak Ozbay: Exact Sciences – Employee.
Thomas Imperiale: Cook Medical – Consultant. Exact Sciences – Advisor or Review Panel Member, Consultant. Exact Sciences Corp – Grant/Research Support.
Mohammad Dehghani: Exact Sciences – Advisor or Review Panel Member, Consultant. Exact Sciences – Consultant.
Michael Dore indicated no relevant financial relationships.
Portia Zaire indicated no relevant financial relationships.
A. Mark Fendrick: AbbVie – Consultant. Agency for Healthcare Research and Quality – Grant/Research Support. Amgen – Consultant. Arnold Ventures – Grant/Research Support. Centers for Medicare and Medicaid Services. – Grant/Research Support. Centivo – Consultant. Community Oncology Association – Consultant. Covered California – Consultant. EmblemHealth – Consultant. Exact Sciences – Consultant. Freedman Health – Consultant. Gary and Mary West Health Policy Center – Grant/Research Support. GRAIL – Consultant. Harvard University – Consultant. Health & Wellness Innovations – Consultant. Health at Scale Technologies – Consultant. MedZed – Consultant. National Pharmaceutical Council – Grant/Research Support. Patient-Centered Outcomes Research Institute – Grant/Research Support. Penguin Pay – Consultant. Pharmaceutical Research and Manufacturers of America – Grant/Research Support. Risalto – Consultant. Robert Wood Johnson Foundation – Grant/Research Support. Sempre Health – Consultant. State of Michigan – Grant/Research Support. State of Minnesota – Consultant. U.S. Department of Defense – Consultant. Virginia Center for Health Innovation – Consultant. Wellthy – Consultant. Zansors – Consultant.
Paul J. Limburg: Exact Sciences – Employee.
Xiting Cao, PhD1, Vahab Vahdat, PhD1, Heather A. Johnson, MLIS1, Gina Thompson, PhD1, Joyce Kong, PharmD2, Irem Betül Koçak, PhD3, Carol Kirshner, MS4, A. Burak Ozbay, PhD1, Thomas Imperiale, MD5, Mohammad Dehghani, PhD6, Michael Dore, MD7, Portia Zaire, PhD8, A. Mark Fendrick, MD8, Paul J. Limburg, MD1. P4808 - FIT-based Colorectal Cancer Screening Completion Rates (Initial Test and Follow-up Colonoscopy) by Health Facility Type: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
