Tuesday Poster Session
Category: Colon
Uncovering the Risk of Herbal Supplements: A First Ever Reported Case of Colitis From <i>Tribulus terrestris</i>
P4692 - Uncovering the Risk of Herbal Supplements: A First Ever Reported Case of Colitis From Tribulus terrestris
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

David Okuampa, MD
Louisiana State University Health
Shreveport, LA
Presenting Author(s)
David Okuampa, MD1, Khadija Khan, MD2, Hammad Qureshi, MD2, Gabrielle Sanford, MD1, Sudha Pandit, MD3
1Louisiana State University Health, Shreveport, LA; 2LSU Health Shreveport, Shreveport, LA; 3Louisiana State University, Shreveport, LA
Introduction: Drug-induced colitis (DiC) is a clinically significant yet often under-recognized condition, with etiologies extending beyond commonly implicated nonsteroidal anti-inflammatory drugs (NSAIDs). Herbal and dietary supplements have emerged as potential contributors. Tribulus terrestris (TT), a plant-based supplement rich in steroidal saponins, is widely promoted for enhancing libido and testosterone. While mild gastrointestinal side effects are documented, colitis due to TT remains largely unreported.
Case Description/
Methods: A 64-year-old male with a medical history of diabetes, hypertension, GERD, erectile dysfunction, and obstructive sleep apnea presented with a 7-day history of profuse, mucoid, and occasionally blood-streaked diarrhea occurring every 10–15 minutes, including nocturnal episodes. Symptoms were accompanied by diffuse abdominal pain and sleep disturbance. He denied recent travel, antibiotic or NSAID use, dietary changes, or infectious contacts. Anti-diarrheals were ineffective.
Initial evaluation, including stool studies for bacterial and parasitic pathogens, Clostridium difficile, and Giardia, was negative. Fecal calprotectin was mildly elevated. Colonoscopy revealed severe ulcerations in the proximal rectum and rectosigmoid colon, with diffuse erythema and loss of vascular pattern extending to the ascending colon. Histology showed a mixed inflammatory infiltrate of eosinophils, neutrophils, and plasma cells—findings consistent with drug-induced or infectious colitis. Imaging ruled out alternative causes. Of note, patient had a normal colonoscopy one year earlier.
A detailed history uncovered chronic use of a TT supplement (45% steroidal saponins) for erectile dysfunction. The supplement was discontinued, and the patient was managed symptomatically. Symptoms resolved within two weeks.
Discussion: Steroidal saponins in TT possess surfactant-like properties that disrupt epithelial integrity and increase intestinal permeability, potentially leading to mucosal injury with prolonged exposure. Despite its reputation as a natural and safe product, TT may pose gastrointestinal risks that are underrecognized in clinical practice.
This is the first reported case of biopsy-confirmed colitis associated with chronic TT use. Clinicians should maintain vigilance for supplement-induced colitis, particularly when evaluating patients with unexplained gastrointestinal symptoms. A comprehensive history of herbal and over-the-counter supplement use is essential for accurate diagnosis and management.
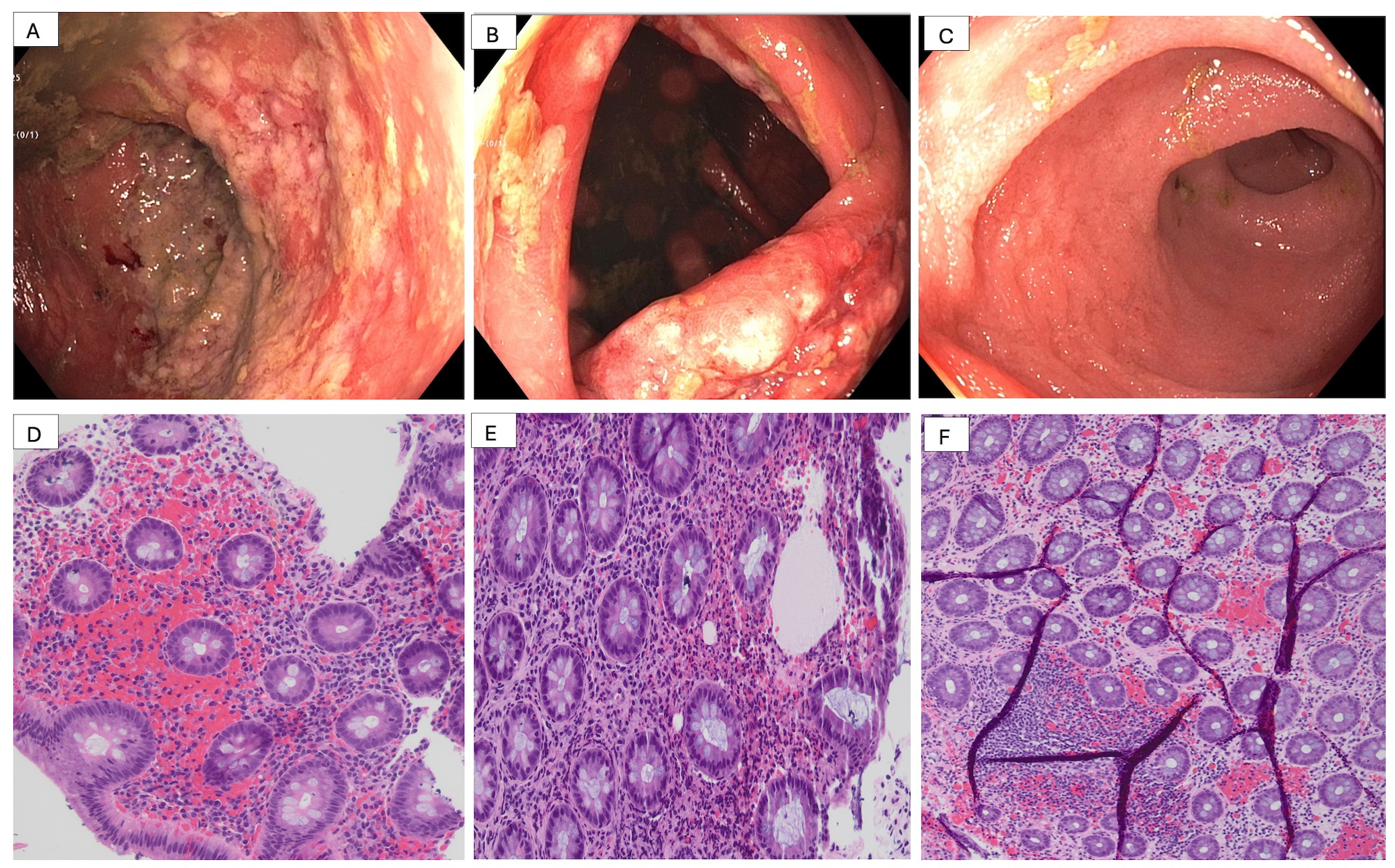
Figure: Figure 1A- Severe, localized edematous, erythematous, friable and ulcerated mucosa rectosigmoid and rectum
Figure 1B - Moderate edematous, erythematous and ulcerated mucosa in the ascending colon
Figure 1C - Normal terminal ileum with no inflammation.
Figure 1D-E- Histological image of focal colitis in ascending and transverse colon respectively
Figure F - Histological image of focal colitis in rectum
Disclosures:
David Okuampa indicated no relevant financial relationships.
Khadija Khan indicated no relevant financial relationships.
Hammad Qureshi indicated no relevant financial relationships.
Gabrielle Sanford indicated no relevant financial relationships.
Sudha Pandit: Medtronic – Product feedback.
David Okuampa, MD1, Khadija Khan, MD2, Hammad Qureshi, MD2, Gabrielle Sanford, MD1, Sudha Pandit, MD3. P4692 - Uncovering the Risk of Herbal Supplements: A First Ever Reported Case of Colitis From <i>Tribulus terrestris</i>, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Louisiana State University Health, Shreveport, LA; 2LSU Health Shreveport, Shreveport, LA; 3Louisiana State University, Shreveport, LA
Introduction: Drug-induced colitis (DiC) is a clinically significant yet often under-recognized condition, with etiologies extending beyond commonly implicated nonsteroidal anti-inflammatory drugs (NSAIDs). Herbal and dietary supplements have emerged as potential contributors. Tribulus terrestris (TT), a plant-based supplement rich in steroidal saponins, is widely promoted for enhancing libido and testosterone. While mild gastrointestinal side effects are documented, colitis due to TT remains largely unreported.
Case Description/
Methods: A 64-year-old male with a medical history of diabetes, hypertension, GERD, erectile dysfunction, and obstructive sleep apnea presented with a 7-day history of profuse, mucoid, and occasionally blood-streaked diarrhea occurring every 10–15 minutes, including nocturnal episodes. Symptoms were accompanied by diffuse abdominal pain and sleep disturbance. He denied recent travel, antibiotic or NSAID use, dietary changes, or infectious contacts. Anti-diarrheals were ineffective.
Initial evaluation, including stool studies for bacterial and parasitic pathogens, Clostridium difficile, and Giardia, was negative. Fecal calprotectin was mildly elevated. Colonoscopy revealed severe ulcerations in the proximal rectum and rectosigmoid colon, with diffuse erythema and loss of vascular pattern extending to the ascending colon. Histology showed a mixed inflammatory infiltrate of eosinophils, neutrophils, and plasma cells—findings consistent with drug-induced or infectious colitis. Imaging ruled out alternative causes. Of note, patient had a normal colonoscopy one year earlier.
A detailed history uncovered chronic use of a TT supplement (45% steroidal saponins) for erectile dysfunction. The supplement was discontinued, and the patient was managed symptomatically. Symptoms resolved within two weeks.
Discussion: Steroidal saponins in TT possess surfactant-like properties that disrupt epithelial integrity and increase intestinal permeability, potentially leading to mucosal injury with prolonged exposure. Despite its reputation as a natural and safe product, TT may pose gastrointestinal risks that are underrecognized in clinical practice.
This is the first reported case of biopsy-confirmed colitis associated with chronic TT use. Clinicians should maintain vigilance for supplement-induced colitis, particularly when evaluating patients with unexplained gastrointestinal symptoms. A comprehensive history of herbal and over-the-counter supplement use is essential for accurate diagnosis and management.

Figure: Figure 1A- Severe, localized edematous, erythematous, friable and ulcerated mucosa rectosigmoid and rectum
Figure 1B - Moderate edematous, erythematous and ulcerated mucosa in the ascending colon
Figure 1C - Normal terminal ileum with no inflammation.
Figure 1D-E- Histological image of focal colitis in ascending and transverse colon respectively
Figure F - Histological image of focal colitis in rectum
Disclosures:
David Okuampa indicated no relevant financial relationships.
Khadija Khan indicated no relevant financial relationships.
Hammad Qureshi indicated no relevant financial relationships.
Gabrielle Sanford indicated no relevant financial relationships.
Sudha Pandit: Medtronic – Product feedback.
David Okuampa, MD1, Khadija Khan, MD2, Hammad Qureshi, MD2, Gabrielle Sanford, MD1, Sudha Pandit, MD3. P4692 - Uncovering the Risk of Herbal Supplements: A First Ever Reported Case of Colitis From <i>Tribulus terrestris</i>, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

