Tuesday Poster Session
Category: Liver
P6056 - Statins, Sartans, Semaglutide, or Stauffer’s? Dissecting Idiosyncratic Drug-Induced Hepatitis in a Complex Patient
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mohamed Belal, MD
McLaren Flint Hospital
Grand Blanc, MI
Presenting Author(s)
Mohamed Belal, MD1, Harneet Ghumman, MD1, Avneet Ghumman, MD1, Mustafa Alnounou, MD2, Harpinder Brar, MD3
1McLaren Flint Hospital, Grand Blanc, MI; 2McLaren Flint Hospital, Flint, MI; 3McLaren Flint Hospital, Toronto, ON, Canada
Introduction: Drug-induced liver injury (DILI) can range from reversible hepatotoxicity to severe liver failure. While Losartan and statins are recognized for their hepatotoxic potential, semaglutide has rare reports of liver-related adverse effects. We present a case of a 54-year-old woman with severe hepatic injury that improved after cessation of Losartan, Rosuvastatin, and Semaglutide.
Case Description/
Methods: A 54-year-old woman presented with acute right upper quadrant pain. Medical history included hypertension, hyperlipidemia, renal cell carcinoma (post-nephrectomy), and recent semaglutide initiation four weeks prior. Home medications were Losartan, Rosuvastatin, and Semaglutide. Labs revealed ALT 1261 U/L, AST 1032 U/L, ALP 357 U/L, bilirubin 1.5 mg/dL, with worsening the next day. Workup, including hepatitis serologies, autoimmune markers, metabolic panels, and imaging, was unremarkable. Liver biopsy showed acute toxic hepatitis with mild inflammation, steatosis, and pigment deposition. Semaglutide was discontinued on admission, and Losartan was stopped on Day 2. Liver enzymes improved, and symptoms resolved.
Discussion: This case exemplifies idiosyncratic drug-induced liver injury (DILI), characterized by a delayed onset, lack of alternative etiologies, and histologic confirmation. While acetaminophen toxicity is the most common cause of DILI in Western populations, idiosyncratic DILI is often linked to antibiotics and herbal supplements. Though statins and sartans are known contributors, GLP-1 receptor agonists like semaglutide are rarely implicated. This case is unique due to the recent semaglutide initiation alongside chronic Losartan and Rosuvastatin use. Improvement after cessation of all three supports a drug-induced etiology. Stauffer syndrome, linked to renal cell carcinoma, was considered but deemed unlikely due to the absence of active malignancy and rapid improvement after drug withdrawal.
Clinicians should consider DILI in patients presenting with unexplained liver enzyme elevations, especially with multiple potentially hepatotoxic medications. Idiosyncratic DILI requires high clinical suspicion and prompt drug cessation to prevent severe outcomes. This case highlights the need for ongoing research into the rare hepatotoxic effects of newer agents, such as semaglutide, and reinforces the importance of careful medication review in complex patients.
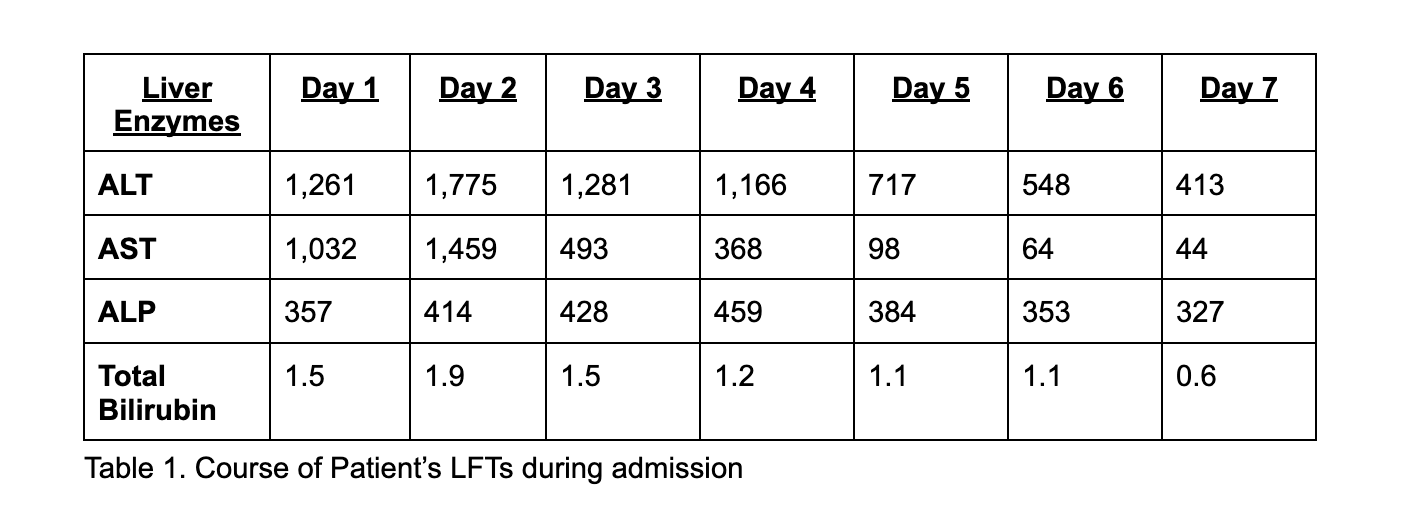
Figure: Table 1. Course of Patient’s LFTs during admission
Disclosures:
Mohamed Belal indicated no relevant financial relationships.
Harneet Ghumman indicated no relevant financial relationships.
Avneet Ghumman indicated no relevant financial relationships.
Mustafa Alnounou indicated no relevant financial relationships.
Harpinder Brar indicated no relevant financial relationships.
Mohamed Belal, MD1, Harneet Ghumman, MD1, Avneet Ghumman, MD1, Mustafa Alnounou, MD2, Harpinder Brar, MD3. P6056 - Statins, Sartans, Semaglutide, or Stauffer’s? Dissecting Idiosyncratic Drug-Induced Hepatitis in a Complex Patient, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1McLaren Flint Hospital, Grand Blanc, MI; 2McLaren Flint Hospital, Flint, MI; 3McLaren Flint Hospital, Toronto, ON, Canada
Introduction: Drug-induced liver injury (DILI) can range from reversible hepatotoxicity to severe liver failure. While Losartan and statins are recognized for their hepatotoxic potential, semaglutide has rare reports of liver-related adverse effects. We present a case of a 54-year-old woman with severe hepatic injury that improved after cessation of Losartan, Rosuvastatin, and Semaglutide.
Case Description/
Methods: A 54-year-old woman presented with acute right upper quadrant pain. Medical history included hypertension, hyperlipidemia, renal cell carcinoma (post-nephrectomy), and recent semaglutide initiation four weeks prior. Home medications were Losartan, Rosuvastatin, and Semaglutide. Labs revealed ALT 1261 U/L, AST 1032 U/L, ALP 357 U/L, bilirubin 1.5 mg/dL, with worsening the next day. Workup, including hepatitis serologies, autoimmune markers, metabolic panels, and imaging, was unremarkable. Liver biopsy showed acute toxic hepatitis with mild inflammation, steatosis, and pigment deposition. Semaglutide was discontinued on admission, and Losartan was stopped on Day 2. Liver enzymes improved, and symptoms resolved.
Discussion: This case exemplifies idiosyncratic drug-induced liver injury (DILI), characterized by a delayed onset, lack of alternative etiologies, and histologic confirmation. While acetaminophen toxicity is the most common cause of DILI in Western populations, idiosyncratic DILI is often linked to antibiotics and herbal supplements. Though statins and sartans are known contributors, GLP-1 receptor agonists like semaglutide are rarely implicated. This case is unique due to the recent semaglutide initiation alongside chronic Losartan and Rosuvastatin use. Improvement after cessation of all three supports a drug-induced etiology. Stauffer syndrome, linked to renal cell carcinoma, was considered but deemed unlikely due to the absence of active malignancy and rapid improvement after drug withdrawal.
Clinicians should consider DILI in patients presenting with unexplained liver enzyme elevations, especially with multiple potentially hepatotoxic medications. Idiosyncratic DILI requires high clinical suspicion and prompt drug cessation to prevent severe outcomes. This case highlights the need for ongoing research into the rare hepatotoxic effects of newer agents, such as semaglutide, and reinforces the importance of careful medication review in complex patients.

Figure: Table 1. Course of Patient’s LFTs during admission
Disclosures:
Mohamed Belal indicated no relevant financial relationships.
Harneet Ghumman indicated no relevant financial relationships.
Avneet Ghumman indicated no relevant financial relationships.
Mustafa Alnounou indicated no relevant financial relationships.
Harpinder Brar indicated no relevant financial relationships.
Mohamed Belal, MD1, Harneet Ghumman, MD1, Avneet Ghumman, MD1, Mustafa Alnounou, MD2, Harpinder Brar, MD3. P6056 - Statins, Sartans, Semaglutide, or Stauffer’s? Dissecting Idiosyncratic Drug-Induced Hepatitis in a Complex Patient, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
