Tuesday Poster Session
Category: Stomach and Spleen
P6311 - Pembrolizumab Versus Chemotherapy in Advanced Gastrointestinal Malignancies: A Meta-Analysis of Efficacy, Toxicity, and PD-L1 Biomarker Stratification
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Sumaiya Tasnim, MBBS2, Aditi Roy, MBBS3, Tonima Ashrafi, 4, Anika Chowdhury, MBBS5, Asif Zamir, MD, FACG6
1DHR Health, Edinburg, Tx, McAllen, TX; 2Bangladesh Medical college, Lynnwood, WA; 3Sher-E-Bangla Medical College Barisal, bangladesh, Redmond, WA; 4bangladesh medical college, Bothell, WA; 5Shaheed Suhrawardy Medical College and Hospital, Port Jefferson, NY; 6DHR Health Gastroenterology, Edinburg, TX
Introduction: Pembrolizumab, an anti–programmed death-1 (PD-1) immune checkpoint inhibitor, has emerged as a treatment option for advanced gastrointestinal (GI) cancers. It targets the PD-1/programmed death-ligand 1 (PD-L1) axis to restore antitumor immune responses. While it may offer superior efficacy and lower systemic toxicity compared to chemotherapy, concerns about immune-mediated adverse events (IMAEs) and inconsistent biomarker stratification remain. This meta-analysis evaluates the therapeutic and adverse event profiles of pembrolizumab relative to chemotherapy, with a focus on PD-L1 biomarker-based subgroup analysis.
Methods: A meta-analysis of four randomized controlled trials (n = 1,059) was conducted, comparing pembrolizumab to standard chemotherapy in advanced gastric and gastroesophageal junction (GEJ) adenocarcinomas. Primary outcomes included objective response rate (ORR), partial response (PR), complete response (CR), stable disease (SD), treatment-related adverse events (TRAEs), and IMAEs. Subgroup analyses were performed based on PD-L1 combined positive score (CPS ≥1 vs < 1). Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated using a random-effects model.
Results: Pembrolizumab was associated with a significant reduction in TRAEs (OR: 0.14; 95% CI: 0.07–0.27) and improvements in ORR (OR: 0.36; 95% CI: 0.24–0.54), PR (OR: 0.32; 95% CI: 0.22–0.47), and SD (OR: 0.58; 95% CI: 0.42–0.79). However, IMAEs were significantly more common (OR: 3.14; 95% CI: 1.89–5.20). Subgroup analysis revealed superior efficacy and lower toxicity in patients with PD-L1 CPS ≥1, whereas patients with CPS < 1 experienced reduced benefit and increased IMAEs.
Discussion: Pembrolizumab improves response rates and lowers systemic toxicity compared to chemotherapy in advanced GI cancers, particularly among patients with PD-L1 CPS ≥1. However, clinicians should be vigilant for immune-mediated adverse events, especially in PD-L1–negative populations. These findings underscore the clinical importance of biomarker-guided therapy selection in GI oncology to optimize outcomes and reduce harm.
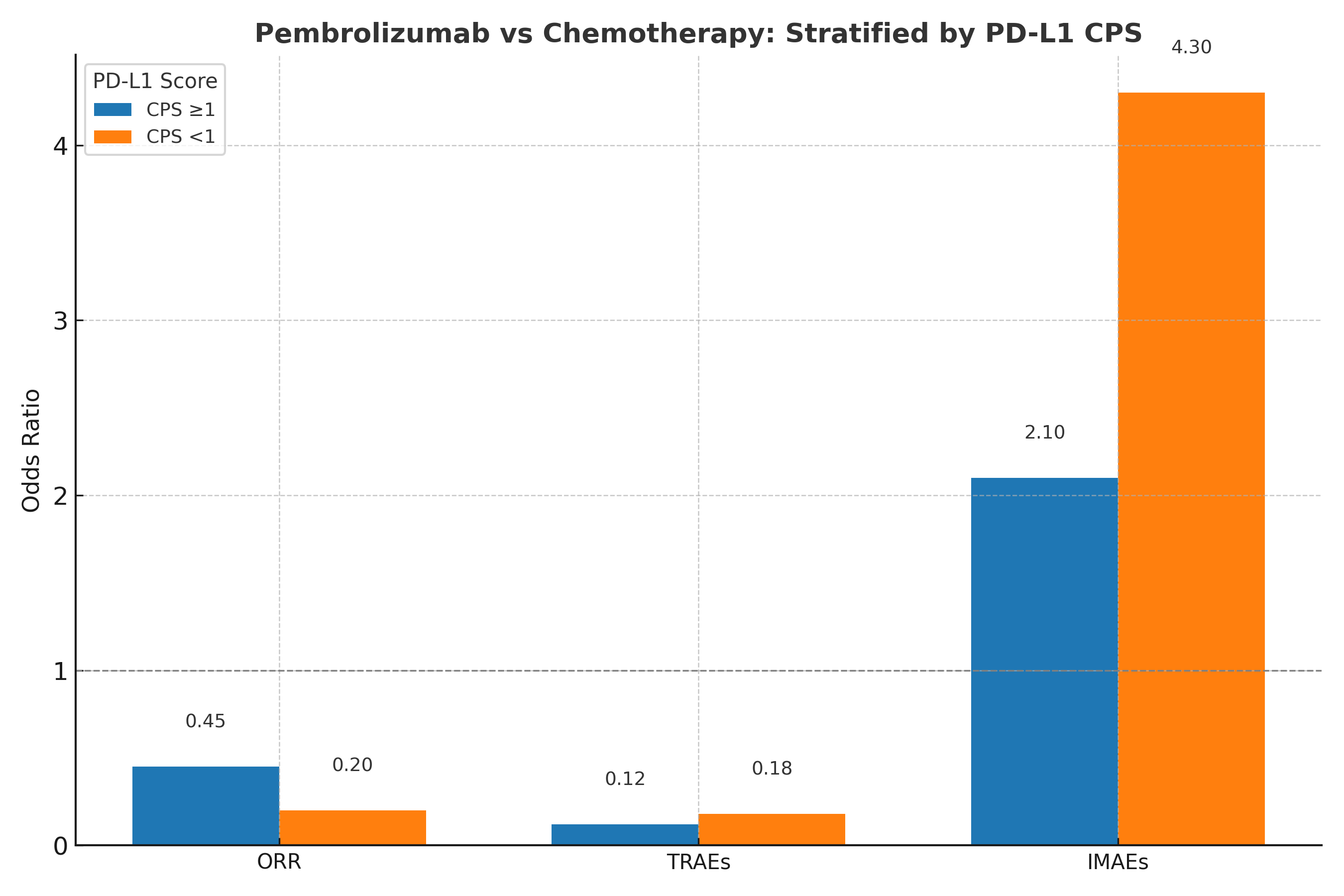
Figure: Figure: Subgroup Analysis of Pembrolizumab vs. Chemotherapy Stratified by PD-L1 Combined Positive Score (CPS)
Odds ratios for overall response rate (ORR), treatment-related adverse events (TRAEs), and immune-mediated adverse events (IMAEs) across PD-L1 CPS ≥1 vs <1 populations.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Sumaiya Tasnim indicated no relevant financial relationships.
Aditi Roy indicated no relevant financial relationships.
Tonima Ashrafi indicated no relevant financial relationships.
Anika Chowdhury indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Sumaiya Tasnim, MBBS2, Aditi Roy, MBBS3, Tonima Ashrafi, 4, Anika Chowdhury, MBBS5, Asif Zamir, MD, FACG6. P6311 - Pembrolizumab Versus Chemotherapy in Advanced Gastrointestinal Malignancies: A Meta-Analysis of Efficacy, Toxicity, and PD-L1 Biomarker Stratification, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2Bangladesh Medical college, Lynnwood, WA; 3Sher-E-Bangla Medical College Barisal, bangladesh, Redmond, WA; 4bangladesh medical college, Bothell, WA; 5Shaheed Suhrawardy Medical College and Hospital, Port Jefferson, NY; 6DHR Health Gastroenterology, Edinburg, TX
Introduction: Pembrolizumab, an anti–programmed death-1 (PD-1) immune checkpoint inhibitor, has emerged as a treatment option for advanced gastrointestinal (GI) cancers. It targets the PD-1/programmed death-ligand 1 (PD-L1) axis to restore antitumor immune responses. While it may offer superior efficacy and lower systemic toxicity compared to chemotherapy, concerns about immune-mediated adverse events (IMAEs) and inconsistent biomarker stratification remain. This meta-analysis evaluates the therapeutic and adverse event profiles of pembrolizumab relative to chemotherapy, with a focus on PD-L1 biomarker-based subgroup analysis.
Methods: A meta-analysis of four randomized controlled trials (n = 1,059) was conducted, comparing pembrolizumab to standard chemotherapy in advanced gastric and gastroesophageal junction (GEJ) adenocarcinomas. Primary outcomes included objective response rate (ORR), partial response (PR), complete response (CR), stable disease (SD), treatment-related adverse events (TRAEs), and IMAEs. Subgroup analyses were performed based on PD-L1 combined positive score (CPS ≥1 vs < 1). Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated using a random-effects model.
Results: Pembrolizumab was associated with a significant reduction in TRAEs (OR: 0.14; 95% CI: 0.07–0.27) and improvements in ORR (OR: 0.36; 95% CI: 0.24–0.54), PR (OR: 0.32; 95% CI: 0.22–0.47), and SD (OR: 0.58; 95% CI: 0.42–0.79). However, IMAEs were significantly more common (OR: 3.14; 95% CI: 1.89–5.20). Subgroup analysis revealed superior efficacy and lower toxicity in patients with PD-L1 CPS ≥1, whereas patients with CPS < 1 experienced reduced benefit and increased IMAEs.
Discussion: Pembrolizumab improves response rates and lowers systemic toxicity compared to chemotherapy in advanced GI cancers, particularly among patients with PD-L1 CPS ≥1. However, clinicians should be vigilant for immune-mediated adverse events, especially in PD-L1–negative populations. These findings underscore the clinical importance of biomarker-guided therapy selection in GI oncology to optimize outcomes and reduce harm.

Figure: Figure: Subgroup Analysis of Pembrolizumab vs. Chemotherapy Stratified by PD-L1 Combined Positive Score (CPS)
Odds ratios for overall response rate (ORR), treatment-related adverse events (TRAEs), and immune-mediated adverse events (IMAEs) across PD-L1 CPS ≥1 vs <1 populations.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Sumaiya Tasnim indicated no relevant financial relationships.
Aditi Roy indicated no relevant financial relationships.
Tonima Ashrafi indicated no relevant financial relationships.
Anika Chowdhury indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Sumaiya Tasnim, MBBS2, Aditi Roy, MBBS3, Tonima Ashrafi, 4, Anika Chowdhury, MBBS5, Asif Zamir, MD, FACG6. P6311 - Pembrolizumab Versus Chemotherapy in Advanced Gastrointestinal Malignancies: A Meta-Analysis of Efficacy, Toxicity, and PD-L1 Biomarker Stratification, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
