Tuesday Poster Session
Category: Stomach and Spleen
Improving <i>Helicobacter pylori</i> Eradication Testing Rates Through a Dedicated Pharmacy Clinic: A Quality Improvement Initiative
P6309 - Improving Helicobacter pylori Eradication Testing Rates Through a Dedicated Pharmacy Clinic: A Quality Improvement Initiative
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Samantha A. Menegas, MD
Duke University Hospital
Durham, NC
Presenting Author(s)
Award: ACG Presidential Poster Award
Samantha A. Menegas, MD1, Sabrina Layne, MD1, Sachiko Oshima, MD1, Collins Mbonu, MD1, Daniel Bernstein, MD1, Rachel Britt, PharmD2, Brian Sullivan, MD1
1Duke University Hospital, Durham, NC; 2Durham VA Medical Center, Durham, NC
Introduction: Helicobacter pylori (HP) infects a third of the United States population and is a major cause of peptic ulcers, malabsorption, and malignancy. After diagnosis and treatment, eradication testing is crucial due to high treatment failure rates, yet studies suggest only about 24% of patients undergo eradication testing. Barriers to testing include fragmented care between gastroenterology (GI) and primary care providers (PCPs), lack of standardized protocols, and adherence challenges.
Methods: This quality improvement study aimed to compare rates of eradication testing and treatment success for HP diagnosed by esophagogastroduodenoscopy (EGD) before and after implementing a dedicated Clinical Pharmacist Practitioner (CPP) at a Veterans Affairs Medical Center. The study compared veterans diagnosed with HP via gastric biopsy between May and October 2023 to those diagnosed during the same period in 2024. A six-month observation period was used to allow for appropriate follow-up. In the pre-intervention period, a mandatory EGD patient results letter was modified to provide "clinical decision support" for HP treatment. In the post-intervention period, the letter was modified to trigger a "clinical referral" to the dedicated CPP clinic for HP management. Chi-square tests were used to compare categorical outcome variables. This study was deemed exempt by the IRB.
Results: There were 601 EGDs in the pre-CPP period (48 HP-positive) and 658 EGDs in the post-CPP period (41 HP-positive). The rate of eradication testing completion increased from 37.5% pre-CPP to 61% post-CPP (p = 0.03). The use of tetracycline-based quadruple therapy increased from 29.2% to 78% (p < 0.001), reflecting concordance with updated guidelines. Indeed, the overall rate of successful eradication improved from 31.3% to 56.1% (p = 0.02) (Table 1). Orders for testing shifted from GI/PCP to CPP-led (76% post-intervention, p < 0.001). Among patients who completed testing, the rate of refractory HP decreased from 16.7% to 8.0% (Table 2).
Discussion: A dedicated CPP clinic significantly improved HP eradication testing rates and streamlined care, with most post-intervention veterans managed by a pharmacist. Centralized follow-up with standardized, guideline-concordant treatment regimens improved adherence and outcomes, demonstrating a scalable model for HP management. We anticipate continued improvement in outcomes after the “wash-in” period with this new model, given rising utilization and satisfaction with the CPP clinic.
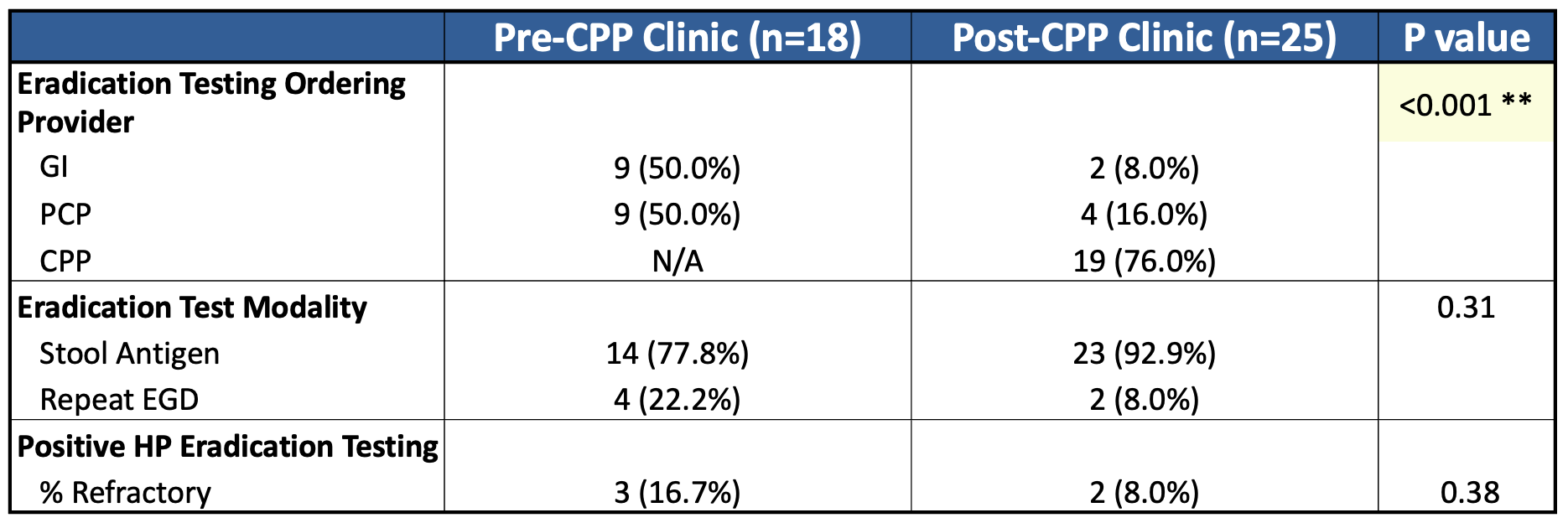
Figure: H. pylori treatment and eradication characteristics among all patients
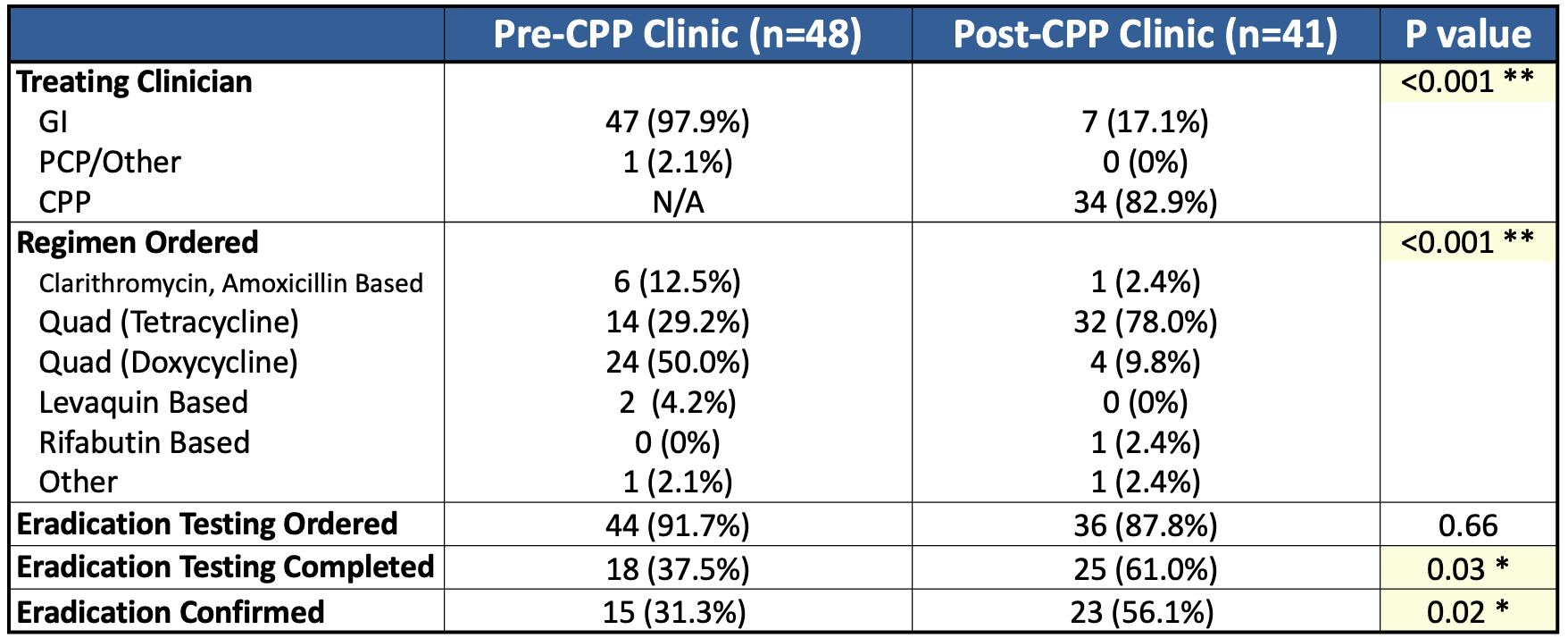
Figure: Additional outcomes among patients who underwent eradication testing
Disclosures:
Samantha Menegas indicated no relevant financial relationships.
Sabrina Layne indicated no relevant financial relationships.
Sachiko Oshima indicated no relevant financial relationships.
Collins Mbonu indicated no relevant financial relationships.
Daniel Bernstein indicated no relevant financial relationships.
Rachel Britt indicated no relevant financial relationships.
Brian Sullivan indicated no relevant financial relationships.
Samantha A. Menegas, MD1, Sabrina Layne, MD1, Sachiko Oshima, MD1, Collins Mbonu, MD1, Daniel Bernstein, MD1, Rachel Britt, PharmD2, Brian Sullivan, MD1. P6309 - Improving <i>Helicobacter pylori</i> Eradication Testing Rates Through a Dedicated Pharmacy Clinic: A Quality Improvement Initiative, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Samantha A. Menegas, MD1, Sabrina Layne, MD1, Sachiko Oshima, MD1, Collins Mbonu, MD1, Daniel Bernstein, MD1, Rachel Britt, PharmD2, Brian Sullivan, MD1
1Duke University Hospital, Durham, NC; 2Durham VA Medical Center, Durham, NC
Introduction: Helicobacter pylori (HP) infects a third of the United States population and is a major cause of peptic ulcers, malabsorption, and malignancy. After diagnosis and treatment, eradication testing is crucial due to high treatment failure rates, yet studies suggest only about 24% of patients undergo eradication testing. Barriers to testing include fragmented care between gastroenterology (GI) and primary care providers (PCPs), lack of standardized protocols, and adherence challenges.
Methods: This quality improvement study aimed to compare rates of eradication testing and treatment success for HP diagnosed by esophagogastroduodenoscopy (EGD) before and after implementing a dedicated Clinical Pharmacist Practitioner (CPP) at a Veterans Affairs Medical Center. The study compared veterans diagnosed with HP via gastric biopsy between May and October 2023 to those diagnosed during the same period in 2024. A six-month observation period was used to allow for appropriate follow-up. In the pre-intervention period, a mandatory EGD patient results letter was modified to provide "clinical decision support" for HP treatment. In the post-intervention period, the letter was modified to trigger a "clinical referral" to the dedicated CPP clinic for HP management. Chi-square tests were used to compare categorical outcome variables. This study was deemed exempt by the IRB.
Results: There were 601 EGDs in the pre-CPP period (48 HP-positive) and 658 EGDs in the post-CPP period (41 HP-positive). The rate of eradication testing completion increased from 37.5% pre-CPP to 61% post-CPP (p = 0.03). The use of tetracycline-based quadruple therapy increased from 29.2% to 78% (p < 0.001), reflecting concordance with updated guidelines. Indeed, the overall rate of successful eradication improved from 31.3% to 56.1% (p = 0.02) (Table 1). Orders for testing shifted from GI/PCP to CPP-led (76% post-intervention, p < 0.001). Among patients who completed testing, the rate of refractory HP decreased from 16.7% to 8.0% (Table 2).
Discussion: A dedicated CPP clinic significantly improved HP eradication testing rates and streamlined care, with most post-intervention veterans managed by a pharmacist. Centralized follow-up with standardized, guideline-concordant treatment regimens improved adherence and outcomes, demonstrating a scalable model for HP management. We anticipate continued improvement in outcomes after the “wash-in” period with this new model, given rising utilization and satisfaction with the CPP clinic.

Figure: H. pylori treatment and eradication characteristics among all patients

Figure: Additional outcomes among patients who underwent eradication testing
Disclosures:
Samantha Menegas indicated no relevant financial relationships.
Sabrina Layne indicated no relevant financial relationships.
Sachiko Oshima indicated no relevant financial relationships.
Collins Mbonu indicated no relevant financial relationships.
Daniel Bernstein indicated no relevant financial relationships.
Rachel Britt indicated no relevant financial relationships.
Brian Sullivan indicated no relevant financial relationships.
Samantha A. Menegas, MD1, Sabrina Layne, MD1, Sachiko Oshima, MD1, Collins Mbonu, MD1, Daniel Bernstein, MD1, Rachel Britt, PharmD2, Brian Sullivan, MD1. P6309 - Improving <i>Helicobacter pylori</i> Eradication Testing Rates Through a Dedicated Pharmacy Clinic: A Quality Improvement Initiative, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


