Monday Poster Session
Category: Biliary/Pancreas
P2288 - Early-Onset Pancreatitis After a Single Dose of Combination Immune Checkpoint Inhibitor Therapy
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bahar Rehan, MD (she/her/hers)
University of Central Florida, HCA Healthcare GME
Gainesville, FL
Presenting Author(s)
Bahar Rehan, MD1, Yeshika Thapa, MD2, Harsimran Kalsi, MD2, Farigol Hakem Zadeh, MD3, Geran Maule, MD1, Carson Creamer, MD3, Tony Brar, MD2
1University of Central Florida, HCA Healthcare GME, Gainesville, FL; 2University of Central Florida, Gainesville, FL; 3HCA Florida Healthcare, Gainesville, FL
Introduction: Immune checkpoint inhibitors (ICIs) like nivolumab and ipilimumab unleash T-cell antitumor activity but can cause immune-related adverse events. Gastrointestinal toxicities particularly colitis and hepatitis are common, whereas pancreatitis is rare (< 1%) and usually appears later, making it easy to miss. This is a rare case of pancreatitis shortly after a single cycle of nivolumab and ipilimumab.
Case Description/
Methods: A 75-year-old woman with a history of left nephrectomy for renal cell carcinoma, prior lung cancer radiation, and an active pelvic malignancy presented with three days of worsening upper abdominal pain radiating to the back, with nausea and vomiting. She had completed pelvic radiation and received her first dose of combined nivolumab and ipilimumab one week prior. She reported poor tolerance of food, fluids, and medications, along with fatigue and low-grade fever. On exam, she was hemodynamically stable but tender in the upper quadrants without peritoneal signs. Labs showed a drop in hemoglobin from 13.9 to 9.0 g/dL, normal white blood cell and platelet counts, mildly elevated lipase at 97 U/L, normal liver function tests, hypomagnesemia, hypophosphatemia, and triglycerides of 205 mg/dL. CT abdomen/pelvis showed chronic pancreatitis with possible mild acute inflammation, prior nephrectomy, and a suspicious sacral lesion. She denied alcohol use, gallstones, prior pancreatitis, or recent procedures. Given the timing after ICI initiation and exclusion of other causes, immune-related pancreatitis was suspected. She received IV fluids, antiemetics, and electrolyte replacement without corticosteroids, and was discharged after symptom improvement.
Discussion: ICIs can cause rare immune-related pancreatitis (< 1%), usually later in treatment, but this patient developed it just one week after her first dose. Nonspecific symptoms (abdominal pain, nausea, vomiting), mild lipase elevation, and imaging suggesting acute-on-chronic pancreatitis, along with exclusion of alcohol, gallstones, hypertriglyceridemia, and procedures, pointed to an immune cause. Though the mechanism is unclear, heightened T-cell activity from combination ICI likely drove early pancreatic inflammation. With limited guidelines for ICI-related pancreatitis, mild cases are managed supportively (IV fluids, antiemetics, electrolytes) without steroids, as in this case. Clinicians should consider pancreatitis even after a single ICI dose and rely on clinical judgment for timely diagnosis and care.
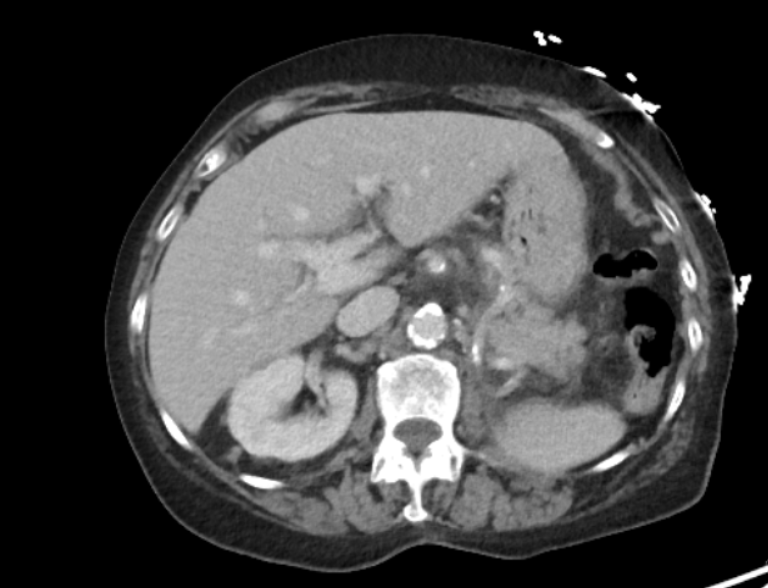
Figure: Contrast-enhanced axial (A) computed tomography images showing mild haziness of the fat around the pancreas.There are calcifications in the head and uncinate process
of the pancreas.
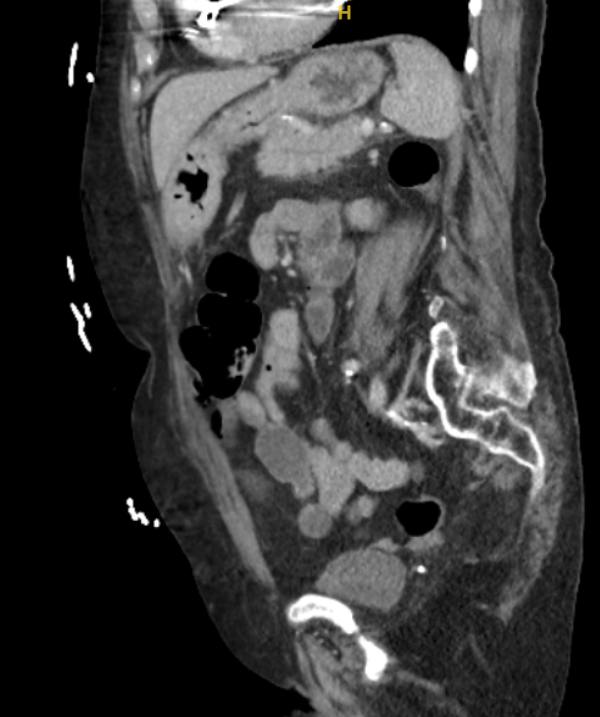
Figure: Contrast-enhanced coronal (B) computed tomography showing mild prominence of the pancreatic duct and common bile duct without overt dilatation. No visible gallstones.
Disclosures:
Bahar Rehan indicated no relevant financial relationships.
Yeshika Thapa indicated no relevant financial relationships.
Harsimran Kalsi indicated no relevant financial relationships.
Farigol Hakem Zadeh indicated no relevant financial relationships.
Geran Maule indicated no relevant financial relationships.
Carson Creamer indicated no relevant financial relationships.
Tony Brar indicated no relevant financial relationships.
Bahar Rehan, MD1, Yeshika Thapa, MD2, Harsimran Kalsi, MD2, Farigol Hakem Zadeh, MD3, Geran Maule, MD1, Carson Creamer, MD3, Tony Brar, MD2. P2288 - Early-Onset Pancreatitis After a Single Dose of Combination Immune Checkpoint Inhibitor Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Central Florida, HCA Healthcare GME, Gainesville, FL; 2University of Central Florida, Gainesville, FL; 3HCA Florida Healthcare, Gainesville, FL
Introduction: Immune checkpoint inhibitors (ICIs) like nivolumab and ipilimumab unleash T-cell antitumor activity but can cause immune-related adverse events. Gastrointestinal toxicities particularly colitis and hepatitis are common, whereas pancreatitis is rare (< 1%) and usually appears later, making it easy to miss. This is a rare case of pancreatitis shortly after a single cycle of nivolumab and ipilimumab.
Case Description/
Methods: A 75-year-old woman with a history of left nephrectomy for renal cell carcinoma, prior lung cancer radiation, and an active pelvic malignancy presented with three days of worsening upper abdominal pain radiating to the back, with nausea and vomiting. She had completed pelvic radiation and received her first dose of combined nivolumab and ipilimumab one week prior. She reported poor tolerance of food, fluids, and medications, along with fatigue and low-grade fever. On exam, she was hemodynamically stable but tender in the upper quadrants without peritoneal signs. Labs showed a drop in hemoglobin from 13.9 to 9.0 g/dL, normal white blood cell and platelet counts, mildly elevated lipase at 97 U/L, normal liver function tests, hypomagnesemia, hypophosphatemia, and triglycerides of 205 mg/dL. CT abdomen/pelvis showed chronic pancreatitis with possible mild acute inflammation, prior nephrectomy, and a suspicious sacral lesion. She denied alcohol use, gallstones, prior pancreatitis, or recent procedures. Given the timing after ICI initiation and exclusion of other causes, immune-related pancreatitis was suspected. She received IV fluids, antiemetics, and electrolyte replacement without corticosteroids, and was discharged after symptom improvement.
Discussion: ICIs can cause rare immune-related pancreatitis (< 1%), usually later in treatment, but this patient developed it just one week after her first dose. Nonspecific symptoms (abdominal pain, nausea, vomiting), mild lipase elevation, and imaging suggesting acute-on-chronic pancreatitis, along with exclusion of alcohol, gallstones, hypertriglyceridemia, and procedures, pointed to an immune cause. Though the mechanism is unclear, heightened T-cell activity from combination ICI likely drove early pancreatic inflammation. With limited guidelines for ICI-related pancreatitis, mild cases are managed supportively (IV fluids, antiemetics, electrolytes) without steroids, as in this case. Clinicians should consider pancreatitis even after a single ICI dose and rely on clinical judgment for timely diagnosis and care.

Figure: Contrast-enhanced axial (A) computed tomography images showing mild haziness of the fat around the pancreas.There are calcifications in the head and uncinate process
of the pancreas.

Figure: Contrast-enhanced coronal (B) computed tomography showing mild prominence of the pancreatic duct and common bile duct without overt dilatation. No visible gallstones.
Disclosures:
Bahar Rehan indicated no relevant financial relationships.
Yeshika Thapa indicated no relevant financial relationships.
Harsimran Kalsi indicated no relevant financial relationships.
Farigol Hakem Zadeh indicated no relevant financial relationships.
Geran Maule indicated no relevant financial relationships.
Carson Creamer indicated no relevant financial relationships.
Tony Brar indicated no relevant financial relationships.
Bahar Rehan, MD1, Yeshika Thapa, MD2, Harsimran Kalsi, MD2, Farigol Hakem Zadeh, MD3, Geran Maule, MD1, Carson Creamer, MD3, Tony Brar, MD2. P2288 - Early-Onset Pancreatitis After a Single Dose of Combination Immune Checkpoint Inhibitor Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
