Monday Poster Session
Category: Esophagus
P2743 - Guideline-Based Deprescription of PPIs: A Quality Improvement Initiative to Reduce Inappropriate PPI Use
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Lahari Kota, MD (she/her/hers)
Hackensack University Medical Center
Secaucus, NJ
Presenting Author(s)
Lahari Kota, MD1, Tyler Schoch, MD2, James Di Palma-Grisi, MD2, Erica DeCecco, MD2, Harinder Bawa, MD3, Jonathan Shammash, MD3, Neha Paralkar, MD3
1Hackensack University Medical Center, Secaucus, NJ; 2Hackensack University Medical Center, Hackensack, NJ; 3Hackensack Meridian Health, Hackensack, NJ
Introduction: The American Gastroenterological Association (AGA) guidelines recommend a routine review of proton pump inhibitor (PPI) use with discontinuation suggested after 8 weeks unless otherwise indicated. However, numerous systematic reviews from the United States have shown chronic PPI use in greater than 20 % of patients without an indication for longterm therapy. While beneficial and well tolerated, PPIs are associated with side effects including C. Difficile infections, vitamin B12 deficiency, osteoporotic fractures, and interactions with commonly used medications. Additionally, PPI misuse contributes to the financial burden of healthcare with an estimated $ 10 billion spent annually between 2002 and 2017. Given this information, we aimed to increase the guideline based deprescription of PPIs in our clinic to 30 % by June 2025.
Methods: This is a quality improvement (QI) project implemented in the Hackensack Meridian Health (HMH) Internal Medicine (IM) academic practice. A thorough presentation was provided to the IM residents which included the following information: guidelines regarding the acute and chronic indications for PPI use, medication side effects, pill burden, patient counseling, and indications for GI referral. An Epic SmartList was created with an algorithm that assisted providers with identifying candidates for PPI deprescription. This Epic Smartlist can be added to clinic notes following a medication reconciliation. The use of the SmartList was tracked with the assistance of the Epic team as well as a manual review to determine rates of appropriate PPI discontinuation in the clinic before and after study initiation.
Results: The study was conducted from December 2024 to June 2025. Pre-intervention data (n = 60) showed a 7 % rate of guideline based PPI deprescription. The post-intervention data (n = 280) showed appropriate discontinuation in 25 % of patients. Secondary outcomes include increases in GI referrals for refractory symptoms by 21 % and appropriate PPI use by 37 %. Additionally, there was a 41 % increase in evaluating the need for PPI use.
Discussion: While this QI project did not achieve the aim of 30 % deprescription in our clinic, it improved patient care by increasing residents’ awareness of the guidelines resulting in decreased pill burden and increased appropriate referrals to a GI specialist for previously overlooked refractory symptoms. This QI project fills an important care gap with significant improvement in guideline based deprescription of PPIs.
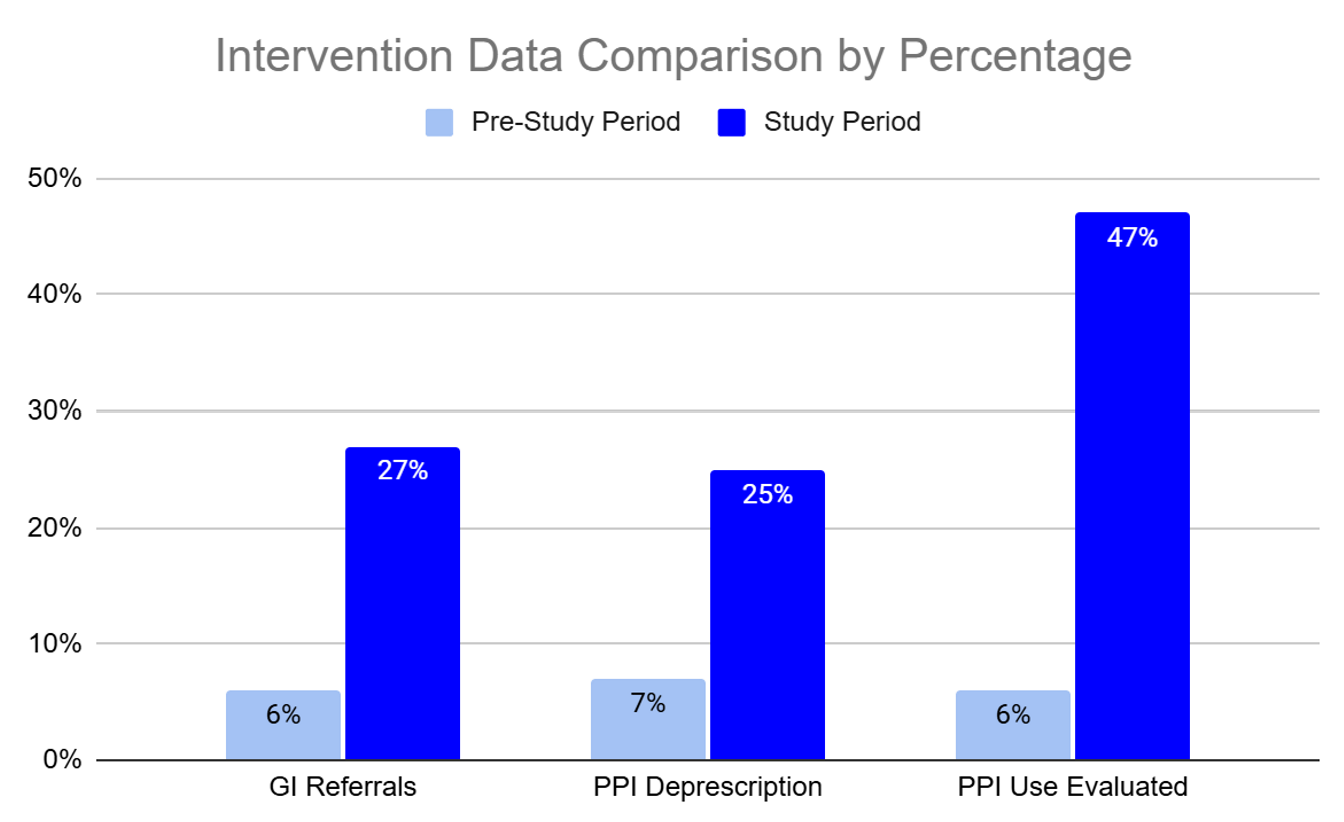
Figure: Pre-intervention and QI intervention data. This compares percent of appropriate GI referrals for refractory symptoms on > 8 weeks of PPI use, guideline-based PPI deprescription, and overall evaluation of the need for PPI use during medication reconciliation.
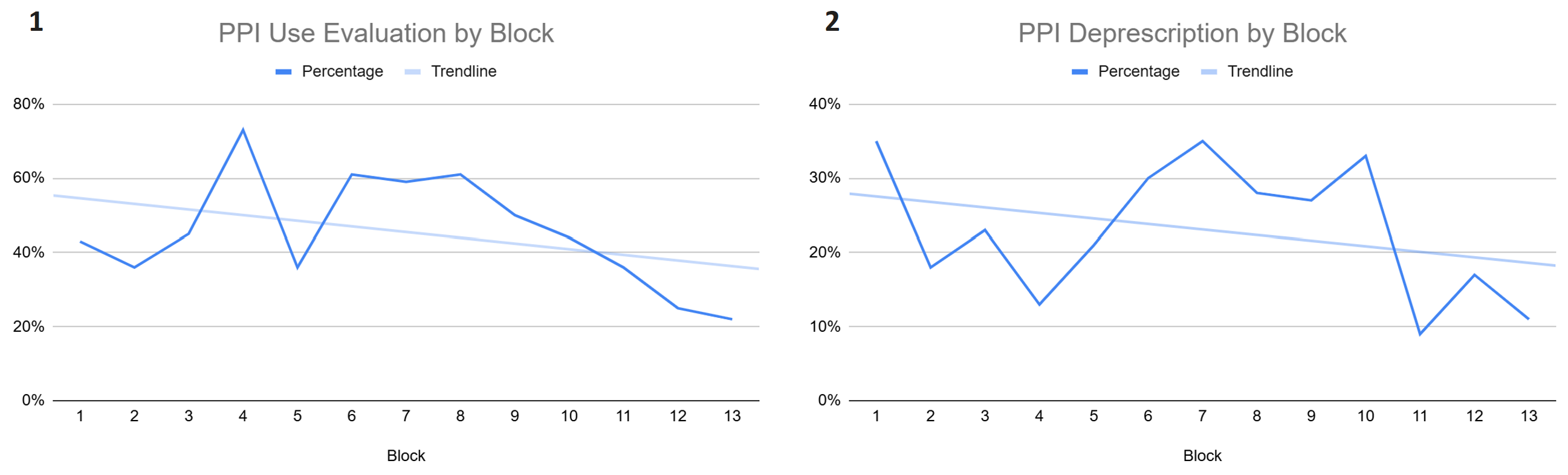
Figure: Figure 1: Evaluation of the appropriateness of PPI use over the study period with each block representing a 2 week period. Figure 2: Guideline-based PPI deprescription over the study period with each block representing a 2 week period.
Disclosures:
Lahari Kota indicated no relevant financial relationships.
Tyler Schoch indicated no relevant financial relationships.
James Di Palma-Grisi indicated no relevant financial relationships.
Erica DeCecco indicated no relevant financial relationships.
Harinder Bawa indicated no relevant financial relationships.
Jonathan Shammash indicated no relevant financial relationships.
Neha Paralkar indicated no relevant financial relationships.
Lahari Kota, MD1, Tyler Schoch, MD2, James Di Palma-Grisi, MD2, Erica DeCecco, MD2, Harinder Bawa, MD3, Jonathan Shammash, MD3, Neha Paralkar, MD3. P2743 - Guideline-Based Deprescription of PPIs: A Quality Improvement Initiative to Reduce Inappropriate PPI Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Hackensack University Medical Center, Secaucus, NJ; 2Hackensack University Medical Center, Hackensack, NJ; 3Hackensack Meridian Health, Hackensack, NJ
Introduction: The American Gastroenterological Association (AGA) guidelines recommend a routine review of proton pump inhibitor (PPI) use with discontinuation suggested after 8 weeks unless otherwise indicated. However, numerous systematic reviews from the United States have shown chronic PPI use in greater than 20 % of patients without an indication for longterm therapy. While beneficial and well tolerated, PPIs are associated with side effects including C. Difficile infections, vitamin B12 deficiency, osteoporotic fractures, and interactions with commonly used medications. Additionally, PPI misuse contributes to the financial burden of healthcare with an estimated $ 10 billion spent annually between 2002 and 2017. Given this information, we aimed to increase the guideline based deprescription of PPIs in our clinic to 30 % by June 2025.
Methods: This is a quality improvement (QI) project implemented in the Hackensack Meridian Health (HMH) Internal Medicine (IM) academic practice. A thorough presentation was provided to the IM residents which included the following information: guidelines regarding the acute and chronic indications for PPI use, medication side effects, pill burden, patient counseling, and indications for GI referral. An Epic SmartList was created with an algorithm that assisted providers with identifying candidates for PPI deprescription. This Epic Smartlist can be added to clinic notes following a medication reconciliation. The use of the SmartList was tracked with the assistance of the Epic team as well as a manual review to determine rates of appropriate PPI discontinuation in the clinic before and after study initiation.
Results: The study was conducted from December 2024 to June 2025. Pre-intervention data (n = 60) showed a 7 % rate of guideline based PPI deprescription. The post-intervention data (n = 280) showed appropriate discontinuation in 25 % of patients. Secondary outcomes include increases in GI referrals for refractory symptoms by 21 % and appropriate PPI use by 37 %. Additionally, there was a 41 % increase in evaluating the need for PPI use.
Discussion: While this QI project did not achieve the aim of 30 % deprescription in our clinic, it improved patient care by increasing residents’ awareness of the guidelines resulting in decreased pill burden and increased appropriate referrals to a GI specialist for previously overlooked refractory symptoms. This QI project fills an important care gap with significant improvement in guideline based deprescription of PPIs.

Figure: Pre-intervention and QI intervention data. This compares percent of appropriate GI referrals for refractory symptoms on > 8 weeks of PPI use, guideline-based PPI deprescription, and overall evaluation of the need for PPI use during medication reconciliation.

Figure: Figure 1: Evaluation of the appropriateness of PPI use over the study period with each block representing a 2 week period. Figure 2: Guideline-based PPI deprescription over the study period with each block representing a 2 week period.
Disclosures:
Lahari Kota indicated no relevant financial relationships.
Tyler Schoch indicated no relevant financial relationships.
James Di Palma-Grisi indicated no relevant financial relationships.
Erica DeCecco indicated no relevant financial relationships.
Harinder Bawa indicated no relevant financial relationships.
Jonathan Shammash indicated no relevant financial relationships.
Neha Paralkar indicated no relevant financial relationships.
Lahari Kota, MD1, Tyler Schoch, MD2, James Di Palma-Grisi, MD2, Erica DeCecco, MD2, Harinder Bawa, MD3, Jonathan Shammash, MD3, Neha Paralkar, MD3. P2743 - Guideline-Based Deprescription of PPIs: A Quality Improvement Initiative to Reduce Inappropriate PPI Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
