Monday Poster Session
Category: General Endoscopy
P2985 - Evaluating Glucagon-Like Peptide 1 Receptor Agonist Use and Retained Gastric Contents: Safety Implications for Upper Endoscopy
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Anneleise Frie, MD
University of Wisconsin Hospitals and Clinics
Madison, WI
Presenting Author(s)
Anneleise Frie, MD1, Anurag Soni, MD2, Richard Lennertz, MD, PhD3, Lucas Skoda, DO3, Jeff Baltus, MS4, Jesse Ellin, MS4, Bryan Krause, PhD3, Deepak Gopal, MD, FACG5
1University of Wisconsin Hospitals and Clinics, Madison, WI; 2University of Wisconsin Hospitals and Clinics, Department of Medicine, Division of Gastroenterology and Hepatology, Madison, WI; 3University of Wisconsin Hospitals and Clinics, Department of Anesthesiology, Madison, WI; 4University of Wisconsin Department of Informatics and Information Technology, Madison, WI; 5University of Wisconsin School of Medicine and Public Health, Madison, WI
Introduction: Glucagon-like peptide 1 receptor agonist (GLP-1RA) use is increasingly common. These agents act on the gastrointestinal tract by delaying gastric emptying. GLP-1RA use increases the odds of having retained gastric contents on esophagogastroduodenoscopy (EGD) two- to nine-fold, but there are mixed data regarding their effects on aspiration risk and procedure completion rates. We evaluated EGD records with and without retained gastric content to discern the risks of GLP-1RA use and confounding factors relevant to procedural completion and patient safety outcomes.
Methods: This single-site retrospective case control study evaluated EGD procedures completed from 1/2014 – 12/2024. Participant demographics, fasting times, comorbid conditions, and medications that could contribute to delayed gastric emptying were collected from the electronic health record (EHR). Univariate analysis was performed to associate these factors with the presence of food on EGD, including the use of GLP-1RA medications. Multivariable regression was performed to measure the effect of GLP-1RA medications adjusting for other factors that may influence gastric emptying.
Results: 42,964 procedures were identified from the EHR and 3,181 were excluded because the EGD was canceled, leaving 30,132 patients who underwent 39,783 EGDs. Mean age 52, BMI 29, female sex 60%, and fasting time of 20 hours. The majority of patients adhered to a fasting time >8 hours pre-EGD (figure 1). 991 EGD exams had solid retained gastric content (2.4%). Out of 201 patients who used a GLP-1RA, 23 had retained gastric content on their EGD (11.4%, OR 5.2; adjusted OR 2.9). EGD exams with retained gastric content were associated with higher comorbid complexity (OR 1.2), diabetes (OR 2.5), or gastroparesis (OR 6.3). Procedural limitations and complications occurred at higher rates with retained gastric contents, including poor visualization (OR 68.9), aborted procedure (OR 25.5), and aspiration of gastric contents (OR 28.7). (Table 1).
Discussion: In our analysis a nearly five-fold increase of retained gastric content on EGD exams with GLP-1RA use was seen (11.4% compared to 2.4%). Increased procedural limitations related to retained gastric contents as well as increased aspiration risk supports ongoing concern for higher peri-procedural risks with GLP-1RA use. The presence of associated confounders supports the recent recommendation by ACG and AGA advocating for an individualized approach to managing GLP-1RA ahead of endoscopy procedures.
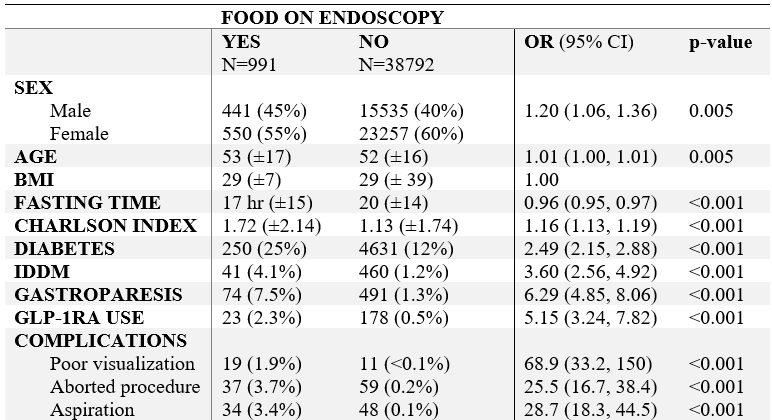
Figure: Table 1: Demographic information of participants and key characteristic factors comparing cohorts of EGDs that showed the presence versus absence of retained solid gastric contents. N with (%) included or mean with (±SD). Univariate analysis with odds ratio, CI, and p-value. (OR = odds ratio, CI = confidence interval, SD = standard deviation)
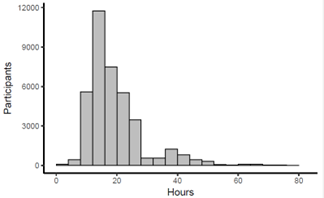
Figure: Figure 1: Histogram representing the frequency of hours participants spent NPO from solid food prior to their EGD.
Disclosures:
Anneleise Frie indicated no relevant financial relationships.
Anurag Soni indicated no relevant financial relationships.
Richard Lennertz indicated no relevant financial relationships.
Lucas Skoda indicated no relevant financial relationships.
Jeff Baltus indicated no relevant financial relationships.
Jesse Ellin indicated no relevant financial relationships.
Bryan Krause indicated no relevant financial relationships.
Deepak Gopal indicated no relevant financial relationships.
Anneleise Frie, MD1, Anurag Soni, MD2, Richard Lennertz, MD, PhD3, Lucas Skoda, DO3, Jeff Baltus, MS4, Jesse Ellin, MS4, Bryan Krause, PhD3, Deepak Gopal, MD, FACG5. P2985 - Evaluating Glucagon-Like Peptide 1 Receptor Agonist Use and Retained Gastric Contents: Safety Implications for Upper Endoscopy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Wisconsin Hospitals and Clinics, Madison, WI; 2University of Wisconsin Hospitals and Clinics, Department of Medicine, Division of Gastroenterology and Hepatology, Madison, WI; 3University of Wisconsin Hospitals and Clinics, Department of Anesthesiology, Madison, WI; 4University of Wisconsin Department of Informatics and Information Technology, Madison, WI; 5University of Wisconsin School of Medicine and Public Health, Madison, WI
Introduction: Glucagon-like peptide 1 receptor agonist (GLP-1RA) use is increasingly common. These agents act on the gastrointestinal tract by delaying gastric emptying. GLP-1RA use increases the odds of having retained gastric contents on esophagogastroduodenoscopy (EGD) two- to nine-fold, but there are mixed data regarding their effects on aspiration risk and procedure completion rates. We evaluated EGD records with and without retained gastric content to discern the risks of GLP-1RA use and confounding factors relevant to procedural completion and patient safety outcomes.
Methods: This single-site retrospective case control study evaluated EGD procedures completed from 1/2014 – 12/2024. Participant demographics, fasting times, comorbid conditions, and medications that could contribute to delayed gastric emptying were collected from the electronic health record (EHR). Univariate analysis was performed to associate these factors with the presence of food on EGD, including the use of GLP-1RA medications. Multivariable regression was performed to measure the effect of GLP-1RA medications adjusting for other factors that may influence gastric emptying.
Results: 42,964 procedures were identified from the EHR and 3,181 were excluded because the EGD was canceled, leaving 30,132 patients who underwent 39,783 EGDs. Mean age 52, BMI 29, female sex 60%, and fasting time of 20 hours. The majority of patients adhered to a fasting time >8 hours pre-EGD (figure 1). 991 EGD exams had solid retained gastric content (2.4%). Out of 201 patients who used a GLP-1RA, 23 had retained gastric content on their EGD (11.4%, OR 5.2; adjusted OR 2.9). EGD exams with retained gastric content were associated with higher comorbid complexity (OR 1.2), diabetes (OR 2.5), or gastroparesis (OR 6.3). Procedural limitations and complications occurred at higher rates with retained gastric contents, including poor visualization (OR 68.9), aborted procedure (OR 25.5), and aspiration of gastric contents (OR 28.7). (Table 1).
Discussion: In our analysis a nearly five-fold increase of retained gastric content on EGD exams with GLP-1RA use was seen (11.4% compared to 2.4%). Increased procedural limitations related to retained gastric contents as well as increased aspiration risk supports ongoing concern for higher peri-procedural risks with GLP-1RA use. The presence of associated confounders supports the recent recommendation by ACG and AGA advocating for an individualized approach to managing GLP-1RA ahead of endoscopy procedures.

Figure: Table 1: Demographic information of participants and key characteristic factors comparing cohorts of EGDs that showed the presence versus absence of retained solid gastric contents. N with (%) included or mean with (±SD). Univariate analysis with odds ratio, CI, and p-value. (OR = odds ratio, CI = confidence interval, SD = standard deviation)

Figure: Figure 1: Histogram representing the frequency of hours participants spent NPO from solid food prior to their EGD.
Disclosures:
Anneleise Frie indicated no relevant financial relationships.
Anurag Soni indicated no relevant financial relationships.
Richard Lennertz indicated no relevant financial relationships.
Lucas Skoda indicated no relevant financial relationships.
Jeff Baltus indicated no relevant financial relationships.
Jesse Ellin indicated no relevant financial relationships.
Bryan Krause indicated no relevant financial relationships.
Deepak Gopal indicated no relevant financial relationships.
Anneleise Frie, MD1, Anurag Soni, MD2, Richard Lennertz, MD, PhD3, Lucas Skoda, DO3, Jeff Baltus, MS4, Jesse Ellin, MS4, Bryan Krause, PhD3, Deepak Gopal, MD, FACG5. P2985 - Evaluating Glucagon-Like Peptide 1 Receptor Agonist Use and Retained Gastric Contents: Safety Implications for Upper Endoscopy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
