Monday Poster Session
Category: IBD
P3377 - Immunotherapy-Induced Celiac Disease: An Uncommon Gastrointestinal Response - A Case Report
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Riddhi Machchhar, DO (she/her/hers)
Hackensack Meridian OUMC
Brick, NJ
Presenting Author(s)
Riddhi Machchhar, DO1, Ana Torres Hernandez, BS2, Ujjwala Jain, MD1, Jyotibala Jain, MD3, Prashant Desai, MD1
1Hackensack Meridian OUMC, Brick, NJ; 2Rowan University School of Osteopathic Medicine, Stratford, NJ; 3Tufts University School of Medicine, Boston, MA
Introduction: Cutaneous Squamous Cell Carcinoma (cSCC) originates from epidermal keratinocytes and can lead to significant local destruction and metastasis. While standard treatments include surgery, immunotherapies like Pembrolizumab and Cemiplimab are gaining prominence for advanced cases. However, these immune checkpoint inhibitors may cause gastrointestinal adverse effects, notably immune-mediated celiac disease, necessitating effective management to improve patient outcomes.
Case Description/
Methods: A 62-year-old woman with a history of skin cancer, primarily managed through monthly excisions and Mohs procedures, sought a second opinion after her last biopsy two years prior. Diagnosed with multiple SCC approximately 20 years ago, she explored systemic therapies, specifically Keytruda (Pembrolizumab) and Cemiplimab (Libtayo). Pathological examination confirmed cSCC on her right calf, prompting a recommendation for immunotherapy due to lesion recurrence. One month into treatment with Cemiplimab, she experienced mild fatigue and nausea but noted improved wound healing. However, by the end of the second month, new lesions emerged, with biopsies indicating residual basal cell carcinoma and a junctional melanocytic nevus with severe atypia. Persistent gastrointestinal symptoms led to a celiac disease diagnosis one year later, resulting in an adjustment of Cemiplimab dosing to every three weeks while maintaining treatment.
Discussion: This case report discusses a rare occurrence of immunotherapy-induced celiac disease in a 62-year-old female patient treated with Cemiplimab for cutaneous squamous cell carcinoma (cSCC). Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, yet they can induce immune-related adverse events (irAEs), particularly affecting the gastrointestinal (GI) tract. The patient's symptoms began with chronic constipation and escalated to severe nausea, diarrhea, and fatigue, which could easily be mistaken for typical GI toxicities associated with ICIs, such as immune-mediated enterocolitis. A thorough differential diagnosis was warranted, leading to a colonoscopy that ruled out colitis, followed by an EGD. Diagnosis of celiac disease usually involves serological testing (notably tTG-IgA and EMA-IgA) and confirmatory intestinal biopsies. The pathophysiology of ICI-induced celiac disease may involve the activation of previously silent celiac disease and hyperactivation of gut T-cells. This underscores the need for vigilant monitoring in patients undergoing treatment with ICIs.
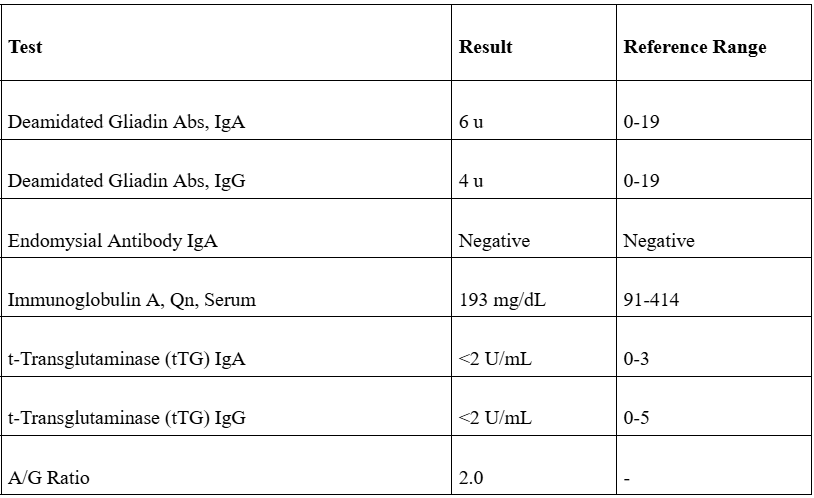
Figure: Table: Diagnostic Labs
Disclosures:
Riddhi Machchhar indicated no relevant financial relationships.
Ana Torres Hernandez indicated no relevant financial relationships.
Ujjwala Jain indicated no relevant financial relationships.
Jyotibala Jain indicated no relevant financial relationships.
Prashant Desai indicated no relevant financial relationships.
Riddhi Machchhar, DO1, Ana Torres Hernandez, BS2, Ujjwala Jain, MD1, Jyotibala Jain, MD3, Prashant Desai, MD1. P3377 - Immunotherapy-Induced Celiac Disease: An Uncommon Gastrointestinal Response - A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Hackensack Meridian OUMC, Brick, NJ; 2Rowan University School of Osteopathic Medicine, Stratford, NJ; 3Tufts University School of Medicine, Boston, MA
Introduction: Cutaneous Squamous Cell Carcinoma (cSCC) originates from epidermal keratinocytes and can lead to significant local destruction and metastasis. While standard treatments include surgery, immunotherapies like Pembrolizumab and Cemiplimab are gaining prominence for advanced cases. However, these immune checkpoint inhibitors may cause gastrointestinal adverse effects, notably immune-mediated celiac disease, necessitating effective management to improve patient outcomes.
Case Description/
Methods: A 62-year-old woman with a history of skin cancer, primarily managed through monthly excisions and Mohs procedures, sought a second opinion after her last biopsy two years prior. Diagnosed with multiple SCC approximately 20 years ago, she explored systemic therapies, specifically Keytruda (Pembrolizumab) and Cemiplimab (Libtayo). Pathological examination confirmed cSCC on her right calf, prompting a recommendation for immunotherapy due to lesion recurrence. One month into treatment with Cemiplimab, she experienced mild fatigue and nausea but noted improved wound healing. However, by the end of the second month, new lesions emerged, with biopsies indicating residual basal cell carcinoma and a junctional melanocytic nevus with severe atypia. Persistent gastrointestinal symptoms led to a celiac disease diagnosis one year later, resulting in an adjustment of Cemiplimab dosing to every three weeks while maintaining treatment.
Discussion: This case report discusses a rare occurrence of immunotherapy-induced celiac disease in a 62-year-old female patient treated with Cemiplimab for cutaneous squamous cell carcinoma (cSCC). Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, yet they can induce immune-related adverse events (irAEs), particularly affecting the gastrointestinal (GI) tract. The patient's symptoms began with chronic constipation and escalated to severe nausea, diarrhea, and fatigue, which could easily be mistaken for typical GI toxicities associated with ICIs, such as immune-mediated enterocolitis. A thorough differential diagnosis was warranted, leading to a colonoscopy that ruled out colitis, followed by an EGD. Diagnosis of celiac disease usually involves serological testing (notably tTG-IgA and EMA-IgA) and confirmatory intestinal biopsies. The pathophysiology of ICI-induced celiac disease may involve the activation of previously silent celiac disease and hyperactivation of gut T-cells. This underscores the need for vigilant monitoring in patients undergoing treatment with ICIs.

Figure: Table: Diagnostic Labs
Disclosures:
Riddhi Machchhar indicated no relevant financial relationships.
Ana Torres Hernandez indicated no relevant financial relationships.
Ujjwala Jain indicated no relevant financial relationships.
Jyotibala Jain indicated no relevant financial relationships.
Prashant Desai indicated no relevant financial relationships.
Riddhi Machchhar, DO1, Ana Torres Hernandez, BS2, Ujjwala Jain, MD1, Jyotibala Jain, MD3, Prashant Desai, MD1. P3377 - Immunotherapy-Induced Celiac Disease: An Uncommon Gastrointestinal Response - A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
