Monday Poster Session
Category: IBD
P3368 - Acneiform Eruption Following Transition From Intravenous to Subcutaneous Vedolizumab Injection in a Patient With Crohn’s Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Harini Raj Shankar Raj, MBBS (she/her/hers)
Allied Digestive Health
North Brunswick, NJ
Presenting Author(s)
Harini Raj Shankar Raj, MBBS, Satya Kastuar, MD, Jhuli Patel, PA-C, MPAS
Allied Digestive Health, North Brunswick, NJ
Introduction: Vedolizumab, an integrin antagonist that inhibits T-cell migration to the gastrointestinal tract, is effective in managing Ulcerative Colitis and Crohn’s disease. While its intravenous (IV) formulation has a well-established safety profile, data on adverse effects following a switch to the subcutaneous (SC) formulation remain limited. Here, we present the first reported case of an acneiform reaction following the transition from intravenous to subcutaneous Vedolizumab in a patient with Crohn's disease.
Case Description/
Methods: A 72-year-old Caucasian male with a history of Crohn’s disease and congenital neutropenia presented with a new-onset facial rash. He was diagnosed with Crohn's disease in 2009 and had been managed with mesalamine, which he discontinued in 2021 in an attempt at conservative management. In 2024, due to worsening symptoms of diarrhea and abdominal pain, a colonoscopy and CT enterography were performed. This revealed an active inflammation in the distal ileum. Vedolizumab therapy was initiated with IV induction doses at weeks 0 and 2. It was switched to SC injection at week 6 for patient convenience. One day after the first SC dose, the patient developed a facial rash. On examination, erythematous papules and pustules were noted over the face. Dermatological evaluation confirmed an acneiform eruption likely secondary to Vedolizumab. Treatment with oral doxycycline, topical clindamycin, and hydroxyzine led to partial resolution within two weeks. The rash recurred following the next SC dose, prompting discontinuation of Vedolizumab. Complete resolution occurred after an 8-week course of oral and topical antibiotics.
Discussion: Vedolizumab targets the α4β7 integrin, blocking its interaction with mucosal addressin cell adhesion molecule-1 in the gastrointestinal tract. Dermatological reactions have been reported in fewer than 5% of patients receiving Vedolizumab. This case highlights a possible formulation-specific immune-mediated skin reaction. While the exact mechanism is unclear, α4β7 expression on epidermotropic T cells may play a role. The pharmacokinetics of SC vedolizumab have also been reported to cause higher drug exposure and more stable trough levels. Management involves withdrawal of the offending agent and symptomatic treatment. Early recognition of this uncommon reaction can prevent prolonged discomfort and allow alternative strategies for the continued management of inflammatory bowel disease.
This abstract was prepared with the assistance of a generative AI tool.
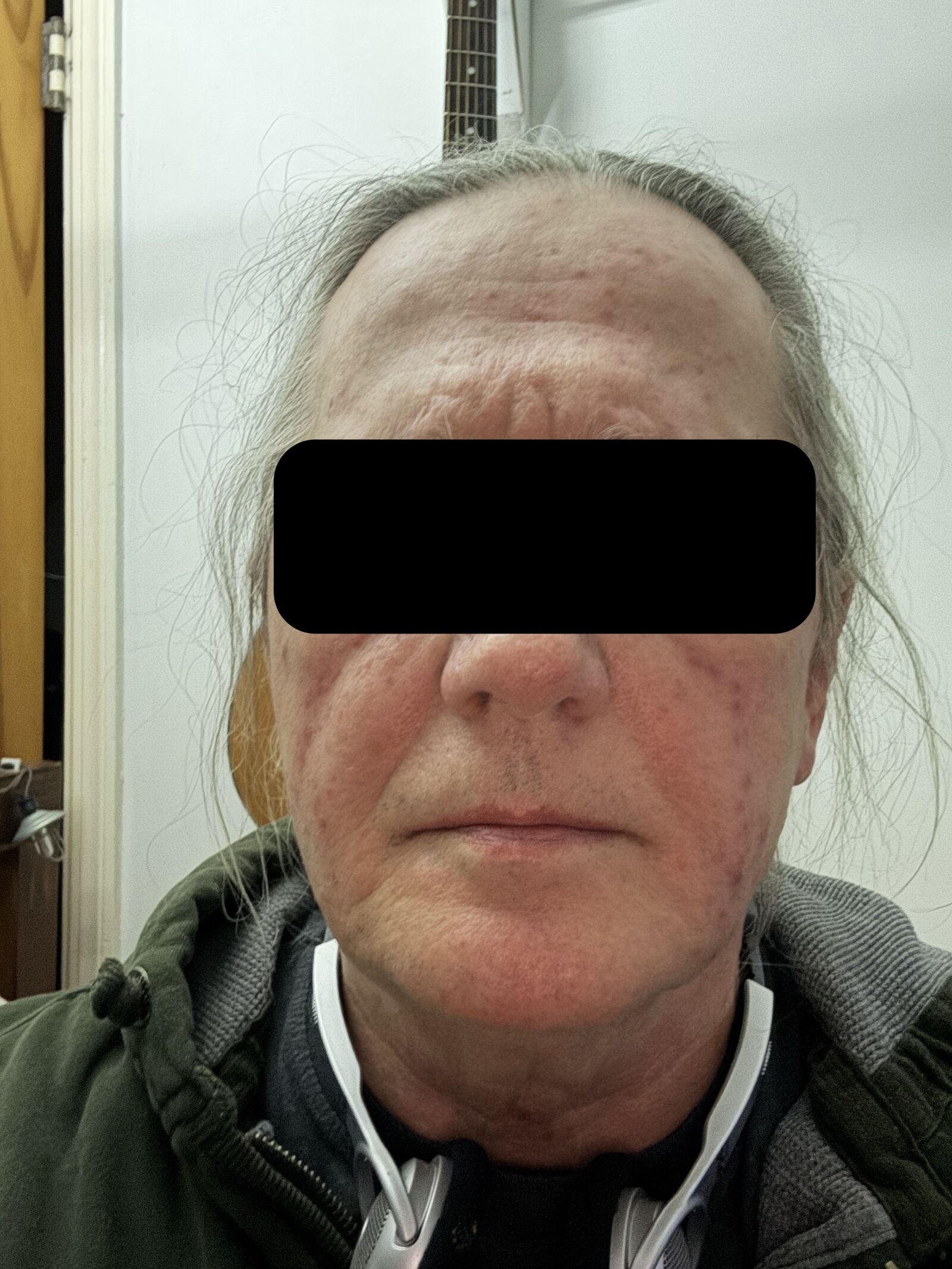
Figure: Multiple erythematous papules and pustules on the forehead, chin, and cheeks
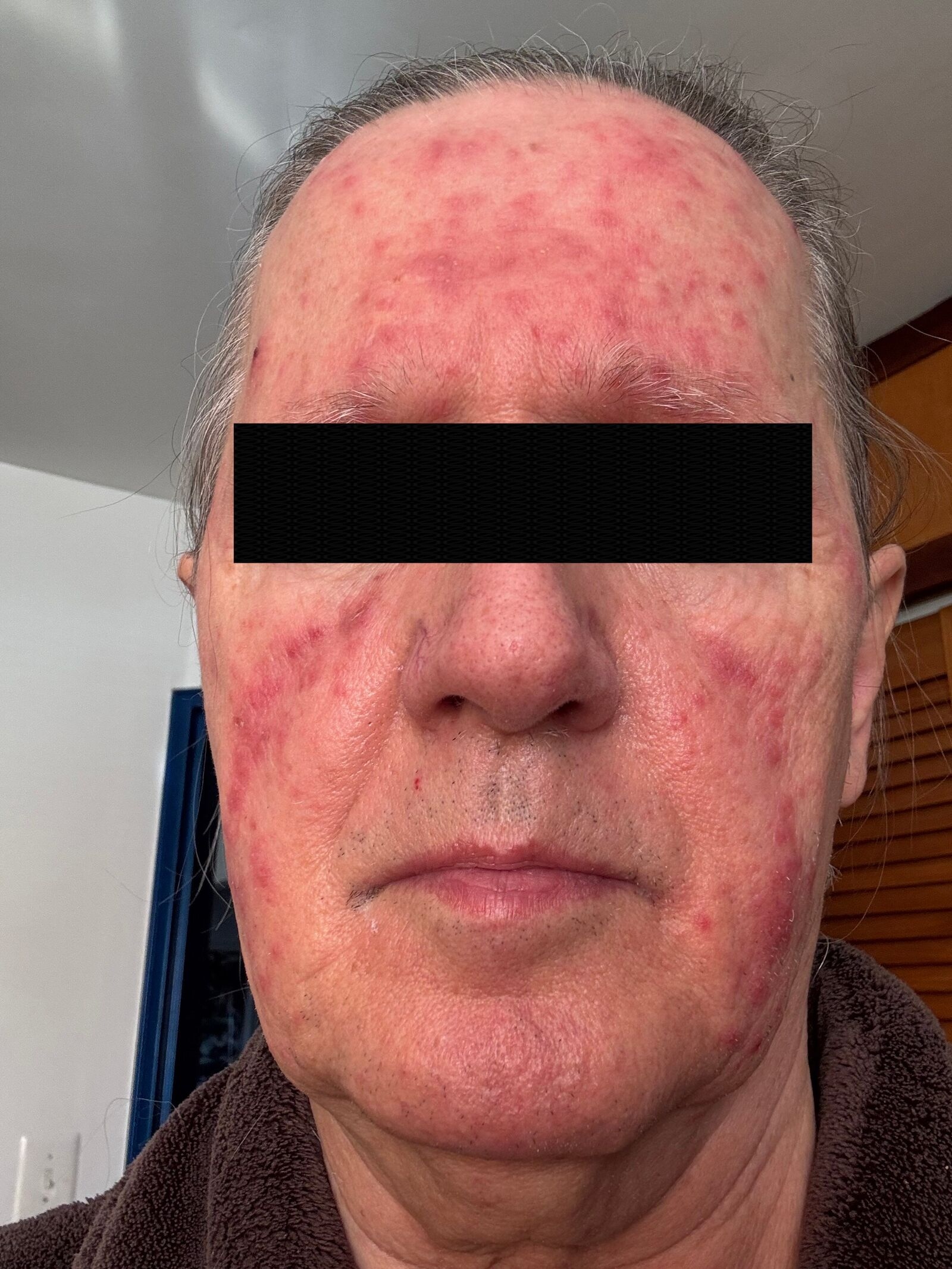
Figure: Complete resolution of the facial lesions after the course of oral and topical antibiotics
Disclosures:
Harini Raj Shankar Raj indicated no relevant financial relationships.
Satya Kastuar indicated no relevant financial relationships.
Jhuli Patel indicated no relevant financial relationships.
Harini Raj Shankar Raj, MBBS, Satya Kastuar, MD, Jhuli Patel, PA-C, MPAS. P3368 - Acneiform Eruption Following Transition From Intravenous to Subcutaneous Vedolizumab Injection in a Patient With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Allied Digestive Health, North Brunswick, NJ
Introduction: Vedolizumab, an integrin antagonist that inhibits T-cell migration to the gastrointestinal tract, is effective in managing Ulcerative Colitis and Crohn’s disease. While its intravenous (IV) formulation has a well-established safety profile, data on adverse effects following a switch to the subcutaneous (SC) formulation remain limited. Here, we present the first reported case of an acneiform reaction following the transition from intravenous to subcutaneous Vedolizumab in a patient with Crohn's disease.
Case Description/
Methods: A 72-year-old Caucasian male with a history of Crohn’s disease and congenital neutropenia presented with a new-onset facial rash. He was diagnosed with Crohn's disease in 2009 and had been managed with mesalamine, which he discontinued in 2021 in an attempt at conservative management. In 2024, due to worsening symptoms of diarrhea and abdominal pain, a colonoscopy and CT enterography were performed. This revealed an active inflammation in the distal ileum. Vedolizumab therapy was initiated with IV induction doses at weeks 0 and 2. It was switched to SC injection at week 6 for patient convenience. One day after the first SC dose, the patient developed a facial rash. On examination, erythematous papules and pustules were noted over the face. Dermatological evaluation confirmed an acneiform eruption likely secondary to Vedolizumab. Treatment with oral doxycycline, topical clindamycin, and hydroxyzine led to partial resolution within two weeks. The rash recurred following the next SC dose, prompting discontinuation of Vedolizumab. Complete resolution occurred after an 8-week course of oral and topical antibiotics.
Discussion: Vedolizumab targets the α4β7 integrin, blocking its interaction with mucosal addressin cell adhesion molecule-1 in the gastrointestinal tract. Dermatological reactions have been reported in fewer than 5% of patients receiving Vedolizumab. This case highlights a possible formulation-specific immune-mediated skin reaction. While the exact mechanism is unclear, α4β7 expression on epidermotropic T cells may play a role. The pharmacokinetics of SC vedolizumab have also been reported to cause higher drug exposure and more stable trough levels. Management involves withdrawal of the offending agent and symptomatic treatment. Early recognition of this uncommon reaction can prevent prolonged discomfort and allow alternative strategies for the continued management of inflammatory bowel disease.
This abstract was prepared with the assistance of a generative AI tool.

Figure: Multiple erythematous papules and pustules on the forehead, chin, and cheeks

Figure: Complete resolution of the facial lesions after the course of oral and topical antibiotics
Disclosures:
Harini Raj Shankar Raj indicated no relevant financial relationships.
Satya Kastuar indicated no relevant financial relationships.
Jhuli Patel indicated no relevant financial relationships.
Harini Raj Shankar Raj, MBBS, Satya Kastuar, MD, Jhuli Patel, PA-C, MPAS. P3368 - Acneiform Eruption Following Transition From Intravenous to Subcutaneous Vedolizumab Injection in a Patient With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
