Monday Poster Session
Category: Interventional Endoscopy
P3511 - Evaluating the Mid-Term Efficacy of Consecutive Hiatal Hernia Repair With Transoral Incisionless Fundoplication for Gastroesophageal Reflux Disease: A Meta-Analysis With 1-Year Follow-Up
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Shahryar Khan, MD
University of Kansas Medical Center
Kansas City, KS
Presenting Author(s)
Shahryar Khan, MD1, Faisal Kamal, MD2, Aamer Ahmad, MD3, Mashal Alam Khan, MBBS4, Falak Hamo, MD5, Hameed Ullah, MD6, Muhammad Waqar Elahi, MD7, Salih Samo, MD, MSci5
1University of Kansas Medical Center, Kansas City, KS; 2Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA; 3Khyber Medical University, Peshawar, North-West Frontier, Pakistan; 4Khyber Medical University, Kansas City, KS; 5University of Kansas Medical Center, Overland Park, KS; 6St. Luke's Hospital, Chesterfield, MO; 7West Virginia University School of Medicine, Morgantown, WV
Introduction: Consecutive hiatal hernia (HH) repair with transoral incisionless fundoplication (cTIF) is an appealing treatment option for refractory gastroesophageal reflux disease (GERD) with a favorable side effect profile compared to the tradition Nissen fundoplication. This meta-analysis aimed to evaluate the mid-term outcomes of cTIF in adult patients with GERD, specifically those with hiatal hernias > 2 cm, who have demonstrated refractoriness to medical therapy.
Methods: A systematic search of PubMed and Embase was conducted up to May 15, 2025. The principal outcome measures, assessed following a minimum 12-month follow-up period, were the GERD-HRQL (Health-Related Quality of Life), RSI (Reflux Symptom Index), and GERSS (Gastroesophageal Reflux Symptom Score) questionnaires. Secondary outcomes included technical success rate, incidence of adverse events, patient satisfaction rate (PSR), and rate of proton pump inhibitors (PPI) discontinuation. A random-effects model was used to calculate the standardized mean difference (SMD) and odds ratio (OR), with statistical significance defined as p < 0.05. Data analysis was performed using RevMan and MedCalc software.
Results: A total of six studies were included, enrolling 602 patients with 494 individuals undergoing cTIF. The mean age of the study population was 58 ± 3.73 years, with a mean BMI of 29 ± 2.2 and a mean HH length of 3.5 ± 0.65 cm. The average follow-up duration was 12.28 ± 2.47 months. Pooled analysis indicated a technical success rate of 100% for cTIF, with an adverse event rate of 4.1%. At 12 months, 75.5% of the patients had ceased PPI use, and the PSR was 76.58%. We detected statistically significant improvements in GERD-HRQL (SMD 4.01, 95% CI 2.35 to 5.67, I2 = 92%, Fig 1), RSI (SMD 2.31, 95% CI 1.69 to 2.92, I2 = 87%, Fig 2), and GERSS (SMD 1.59, 95% CI 1.31 to 1.87, I2 = 0%, Fig 3) following cTIF Although two studies comparing cTIF with surgery alone at 12 months suggested a trend towards increased PPI cessation in the cTIF group (OR 1.69, p = 0.09, I2 = 0%), this difference was not statistically significant.
Discussion: cTIF is technically feasible and demonstrates promising outcomes, as evidenced by significant improvements in mid-term GERD-related scores. Additional well-designed comparative studies across multiple centers are required to validate these observations.
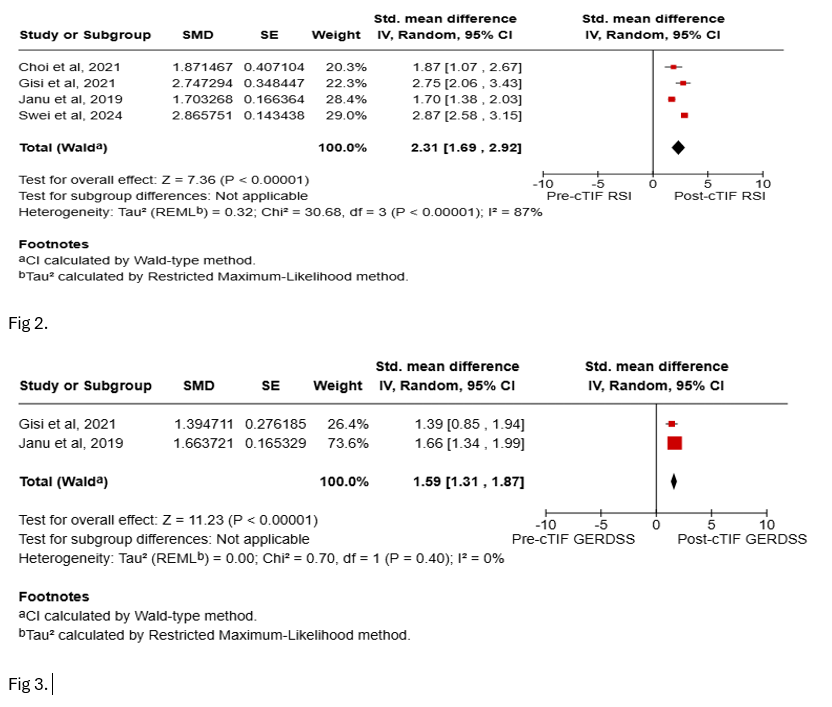
Figure: Fig 1. Forest plot of GERD-HRQL (Health-Related Quality of Life) scores pre- and post- post-cTIF.
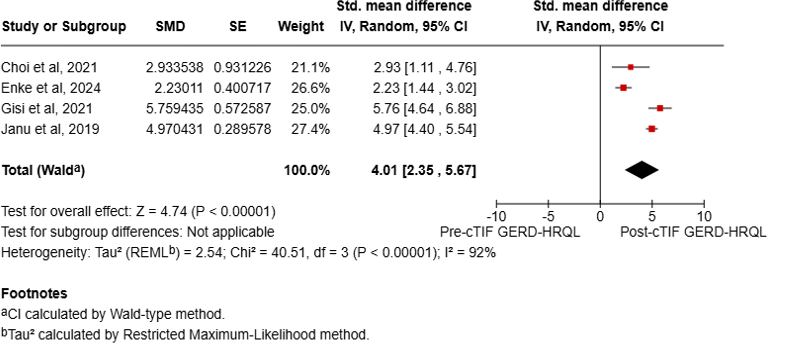
Figure: Fig 2. Forest plot of RSI (Reflux Symptom Index) scores pre- and post-cTIF; Fig 3. Forest plot of GERSS (Gastroesophageal Reflux Symptom Score) pre- and post- post-cTIF.
Disclosures:
Shahryar Khan indicated no relevant financial relationships.
Faisal Kamal indicated no relevant financial relationships.
Aamer Ahmad indicated no relevant financial relationships.
Mashal Alam Khan indicated no relevant financial relationships.
Falak Hamo indicated no relevant financial relationships.
Hameed Ullah indicated no relevant financial relationships.
Muhammad Waqar Elahi indicated no relevant financial relationships.
Salih Samo: Castle Biosciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. EndoGastric Solutions – Speakers Bureau. Evoke – Speakers Bureau. Medtronic – Speakers Bureau. Phathom – Speakers Bureau. Sanofi – Advisory Committee/Board Member, Speakers Bureau. Takeda – Speakers Bureau.
Shahryar Khan, MD1, Faisal Kamal, MD2, Aamer Ahmad, MD3, Mashal Alam Khan, MBBS4, Falak Hamo, MD5, Hameed Ullah, MD6, Muhammad Waqar Elahi, MD7, Salih Samo, MD, MSci5. P3511 - Evaluating the Mid-Term Efficacy of Consecutive Hiatal Hernia Repair With Transoral Incisionless Fundoplication for Gastroesophageal Reflux Disease: A Meta-Analysis With 1-Year Follow-Up, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Kansas Medical Center, Kansas City, KS; 2Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA; 3Khyber Medical University, Peshawar, North-West Frontier, Pakistan; 4Khyber Medical University, Kansas City, KS; 5University of Kansas Medical Center, Overland Park, KS; 6St. Luke's Hospital, Chesterfield, MO; 7West Virginia University School of Medicine, Morgantown, WV
Introduction: Consecutive hiatal hernia (HH) repair with transoral incisionless fundoplication (cTIF) is an appealing treatment option for refractory gastroesophageal reflux disease (GERD) with a favorable side effect profile compared to the tradition Nissen fundoplication. This meta-analysis aimed to evaluate the mid-term outcomes of cTIF in adult patients with GERD, specifically those with hiatal hernias > 2 cm, who have demonstrated refractoriness to medical therapy.
Methods: A systematic search of PubMed and Embase was conducted up to May 15, 2025. The principal outcome measures, assessed following a minimum 12-month follow-up period, were the GERD-HRQL (Health-Related Quality of Life), RSI (Reflux Symptom Index), and GERSS (Gastroesophageal Reflux Symptom Score) questionnaires. Secondary outcomes included technical success rate, incidence of adverse events, patient satisfaction rate (PSR), and rate of proton pump inhibitors (PPI) discontinuation. A random-effects model was used to calculate the standardized mean difference (SMD) and odds ratio (OR), with statistical significance defined as p < 0.05. Data analysis was performed using RevMan and MedCalc software.
Results: A total of six studies were included, enrolling 602 patients with 494 individuals undergoing cTIF. The mean age of the study population was 58 ± 3.73 years, with a mean BMI of 29 ± 2.2 and a mean HH length of 3.5 ± 0.65 cm. The average follow-up duration was 12.28 ± 2.47 months. Pooled analysis indicated a technical success rate of 100% for cTIF, with an adverse event rate of 4.1%. At 12 months, 75.5% of the patients had ceased PPI use, and the PSR was 76.58%. We detected statistically significant improvements in GERD-HRQL (SMD 4.01, 95% CI 2.35 to 5.67, I2 = 92%, Fig 1), RSI (SMD 2.31, 95% CI 1.69 to 2.92, I2 = 87%, Fig 2), and GERSS (SMD 1.59, 95% CI 1.31 to 1.87, I2 = 0%, Fig 3) following cTIF Although two studies comparing cTIF with surgery alone at 12 months suggested a trend towards increased PPI cessation in the cTIF group (OR 1.69, p = 0.09, I2 = 0%), this difference was not statistically significant.
Discussion: cTIF is technically feasible and demonstrates promising outcomes, as evidenced by significant improvements in mid-term GERD-related scores. Additional well-designed comparative studies across multiple centers are required to validate these observations.

Figure: Fig 1. Forest plot of GERD-HRQL (Health-Related Quality of Life) scores pre- and post- post-cTIF.

Figure: Fig 2. Forest plot of RSI (Reflux Symptom Index) scores pre- and post-cTIF; Fig 3. Forest plot of GERSS (Gastroesophageal Reflux Symptom Score) pre- and post- post-cTIF.
Disclosures:
Shahryar Khan indicated no relevant financial relationships.
Faisal Kamal indicated no relevant financial relationships.
Aamer Ahmad indicated no relevant financial relationships.
Mashal Alam Khan indicated no relevant financial relationships.
Falak Hamo indicated no relevant financial relationships.
Hameed Ullah indicated no relevant financial relationships.
Muhammad Waqar Elahi indicated no relevant financial relationships.
Salih Samo: Castle Biosciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. EndoGastric Solutions – Speakers Bureau. Evoke – Speakers Bureau. Medtronic – Speakers Bureau. Phathom – Speakers Bureau. Sanofi – Advisory Committee/Board Member, Speakers Bureau. Takeda – Speakers Bureau.
Shahryar Khan, MD1, Faisal Kamal, MD2, Aamer Ahmad, MD3, Mashal Alam Khan, MBBS4, Falak Hamo, MD5, Hameed Ullah, MD6, Muhammad Waqar Elahi, MD7, Salih Samo, MD, MSci5. P3511 - Evaluating the Mid-Term Efficacy of Consecutive Hiatal Hernia Repair With Transoral Incisionless Fundoplication for Gastroesophageal Reflux Disease: A Meta-Analysis With 1-Year Follow-Up, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

