Tuesday Poster Session
Category: Liver
P6044 - Alpha-1 Antitrypsin Deficiency: The PiMZ Phenotype
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Muaaz Masood, MD
Division of Gastroenterology and Hepatology, Center for Digestive Health, Virginia Mason Franciscan Health
Seattle, WA
Presenting Author(s)
Muaaz Masood, MD1, Christopher Gault, MD2, Randeep Kaur, MD2
1Division of Gastroenterology and Hepatology, Center for Digestive Health, Virginia Mason Franciscan Health, Seattle, WA; 2Virginia Mason Franciscan Health, Seattle, WA
Introduction: Alpha-1 antitrypsin (A1A) deficiency is an autosomal recessive disease due to mutations in the SERPINA1 gene. We report a rare case of a patient with pancreaticobiliary abnormalities who was found to have decompensated cirrhosis, A1A deficiency on liver biopsy, a heterozygous phenotype PiMZ and normal serum A1A levels.
Case Description/
Methods: A 73-year-old female with a history of bronchitis, and a family history of A1A deficiency in her sister, presented to the clinic to establish care for cirrhosis. She reportedly had jaundice 1 year prior and was found to have elevated liver enzymes as well as an elevated CA 19-9 of 249 U/mL. She was hospitalized for the work-up and management of hepatic encephalopathy (HE). She denied alcohol or tobacco use. No risk factors for MASLD were noted. CT abdomen with contrast revealed a nodular liver, biliary and pancreatic ductal dilation, and new ascites. Initial ERCP revealed a common bile duct (CBD) stricture requiring stent placement with a follow-up ERCP demonstrating normal bile ducts and main pancreatic duct (PD) dilation. Biopsies of the CBD revealed acute and chronic inflammation. A liver biopsy revealed features of A1A deficiency (Figure 1). Pancreatic duct fluid sampling revealed mucinous IPMN. She was discharged on lactulose for HE and diuretics for her ascites.
Outpatient work-up was normal except for an INR of 1.2, AST of 41 U/L and alkaline phosphatase of 159 U/L. A1A level was normal at 126 mg/dL. A1A genotype was heterozygous for the Z allele which suggested that she is a carrier of A1A deficiency.
Discussion: The prevalence of the PiMZ phenotype has been reported to be 2-7.1% (1). The risk of liver disease and hepatic decompensation may be increased with the PiMZ phenotype (2). Patients are more likely to develop cirrhosis with advanced age and the PiMZ phenotype. The PiMZ variant has been noted to be a risk factor for cirrhosis in patients with MASLD, alcohol use and cystic fibrosis (3). Additionally, PiMZ has been associated with higher serum transaminases and A1A inclusions in the liver compared to those without the PiMZ variant (4). As in our case, heterozygotes for the Z allele may have serum A1AT levels that overlap with the normal ranges. Patients with the PiMZ phenotype should be counseled regarding their risk of liver disease. Additional studies are warranted to explore outcomes with the PiMZ phenotype.
1 Strnad P. NEJM. 2020
2 Chen VL. JHEP Rep. 2022
3 Strnad P. Gut. 2019
4 Schneider CV. Gastroenterology. 2020
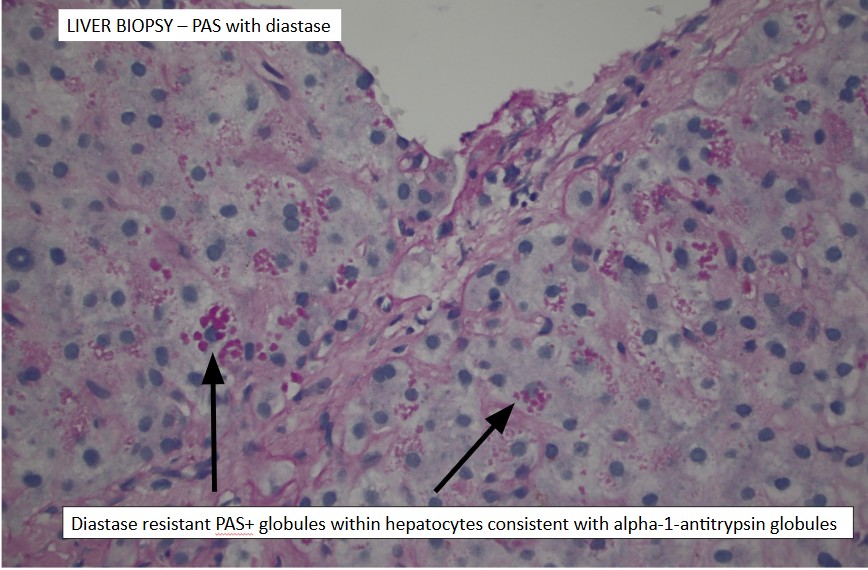
Figure: Figure 1. An endoscopic ultrasound-guided liver biopsy revealed hepatocytes with cytoplasmic Periodic Acid-Schiff diastase-positive globules compatible with alpha-1 antitrypsin deficiency in addition to cirrhosis, no steatosis or iron deposition.
Disclosures:
Muaaz Masood indicated no relevant financial relationships.
Christopher Gault indicated no relevant financial relationships.
Randeep Kaur indicated no relevant financial relationships.
Muaaz Masood, MD1, Christopher Gault, MD2, Randeep Kaur, MD2. P6044 - Alpha-1 Antitrypsin Deficiency: The PiMZ Phenotype, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology and Hepatology, Center for Digestive Health, Virginia Mason Franciscan Health, Seattle, WA; 2Virginia Mason Franciscan Health, Seattle, WA
Introduction: Alpha-1 antitrypsin (A1A) deficiency is an autosomal recessive disease due to mutations in the SERPINA1 gene. We report a rare case of a patient with pancreaticobiliary abnormalities who was found to have decompensated cirrhosis, A1A deficiency on liver biopsy, a heterozygous phenotype PiMZ and normal serum A1A levels.
Case Description/
Methods: A 73-year-old female with a history of bronchitis, and a family history of A1A deficiency in her sister, presented to the clinic to establish care for cirrhosis. She reportedly had jaundice 1 year prior and was found to have elevated liver enzymes as well as an elevated CA 19-9 of 249 U/mL. She was hospitalized for the work-up and management of hepatic encephalopathy (HE). She denied alcohol or tobacco use. No risk factors for MASLD were noted. CT abdomen with contrast revealed a nodular liver, biliary and pancreatic ductal dilation, and new ascites. Initial ERCP revealed a common bile duct (CBD) stricture requiring stent placement with a follow-up ERCP demonstrating normal bile ducts and main pancreatic duct (PD) dilation. Biopsies of the CBD revealed acute and chronic inflammation. A liver biopsy revealed features of A1A deficiency (Figure 1). Pancreatic duct fluid sampling revealed mucinous IPMN. She was discharged on lactulose for HE and diuretics for her ascites.
Outpatient work-up was normal except for an INR of 1.2, AST of 41 U/L and alkaline phosphatase of 159 U/L. A1A level was normal at 126 mg/dL. A1A genotype was heterozygous for the Z allele which suggested that she is a carrier of A1A deficiency.
Discussion: The prevalence of the PiMZ phenotype has been reported to be 2-7.1% (1). The risk of liver disease and hepatic decompensation may be increased with the PiMZ phenotype (2). Patients are more likely to develop cirrhosis with advanced age and the PiMZ phenotype. The PiMZ variant has been noted to be a risk factor for cirrhosis in patients with MASLD, alcohol use and cystic fibrosis (3). Additionally, PiMZ has been associated with higher serum transaminases and A1A inclusions in the liver compared to those without the PiMZ variant (4). As in our case, heterozygotes for the Z allele may have serum A1AT levels that overlap with the normal ranges. Patients with the PiMZ phenotype should be counseled regarding their risk of liver disease. Additional studies are warranted to explore outcomes with the PiMZ phenotype.
1 Strnad P. NEJM. 2020
2 Chen VL. JHEP Rep. 2022
3 Strnad P. Gut. 2019
4 Schneider CV. Gastroenterology. 2020

Figure: Figure 1. An endoscopic ultrasound-guided liver biopsy revealed hepatocytes with cytoplasmic Periodic Acid-Schiff diastase-positive globules compatible with alpha-1 antitrypsin deficiency in addition to cirrhosis, no steatosis or iron deposition.
Disclosures:
Muaaz Masood indicated no relevant financial relationships.
Christopher Gault indicated no relevant financial relationships.
Randeep Kaur indicated no relevant financial relationships.
Muaaz Masood, MD1, Christopher Gault, MD2, Randeep Kaur, MD2. P6044 - Alpha-1 Antitrypsin Deficiency: The PiMZ Phenotype, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
