Tuesday Poster Session
Category: Liver
P6119 - First Fatal Case Report of Post-COVID-19 Cholangiopathy in a Liver Transplant Recipient
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aryanna Sousa, MD, MPH (she/her/hers)
Rush University Medical Center
Providence, RI
Presenting Author(s)
Aryanna Sousa, MD, MPH1, Giovannie Isaac-Coss, MD2, Edie Chan, MD2, Lloyd Brown, MD2, Nathalie Sela, MD2, John Klein, MD2, Justin Mitchell, MD2, Nancy Reau, MD, FACG2
1Rush University Medical Center, Providence, RI; 2Rush University Medical Center, Chicago, IL
Introduction: Post-COVID cholangiopathy (PCC) is a rare variant of secondary sclerosing cholangitis, typically arising after severe COVID-19, particularly in ICU patients or those requiring prolonged ventilation. It is marked by rapid biliary injury, dramatic elevations in ALP and bilirubin, and can progress to liver failure. PCC affects < 0.1% of hospitalized COVID-19 patients and carries a 30–50% mortality. In liver transplant recipients, it can lead to significant graft dysfunction or loss.
Case Description/
Methods: We report a case of a 67-year-old woman with type 2 diabetes, COPD, emphysema, and alpha-1 antitrypsin deficiency-related cirrhosis who underwent liver transplantation. She was maintained on cyclosporin due to tacrolimus-induced neurotoxicity and experienced post-transplant biliary strictures managed with ERCPs and stents, for which she received Augmentin for prophylaxis at least 3 times.
Her bilirubin remained ≤1.9 mg/dL until post-op month 10. At month 11, she contracted COVID-19, for which she was previously vaccinated. Symptoms were mild, and she recovered at home without treatment. Meanwhile, she also completed a two-week course of Augmentin for otitis media. By month 12, on routine lab check, bilirubin sharply rose to 8.4 mg/dL, with GGT persistently ranging at 200–300 U/L. Repeat ERCPs showed no new strictures. Bilirubin continued to rise. Ursodiol was initiated, and immunosuppression was switched back to tacrolimus, though levels remained therapeutic and there was no clinical improvement.
At post-op month 13, she was admitted with acute liver failure and shock. Liver biopsy revealed mild rejection, severe cholestasis, and focal lymphocytic cholangitis, without signs of drug-induced liver injury (DILI). Due to frailty, she was not re-listed for transplant. She died four days after being transferred for a second opinion. Final labs: bilirubin 55.3 mg/dL, ALP 293 U/L, AST 69 U/L, ALT 43 U/L, INR 1.6.
Discussion: This is the first reported case of PCC in a liver transplant recipient. Uniquely, the patient developed PCC after a mild COVID-19 illness. Chronic immunosuppression may have contributed to the severity and fatality. SARS-CoV-2 directly injures cholangiocytes via ACE2 receptors. Although DILI was not evident on biopsy, repeated Augmentin use may have played a role. Given its high mortality, early recognition of PCC in transplant patients is critical to consider re-listing before irreversible graft loss.
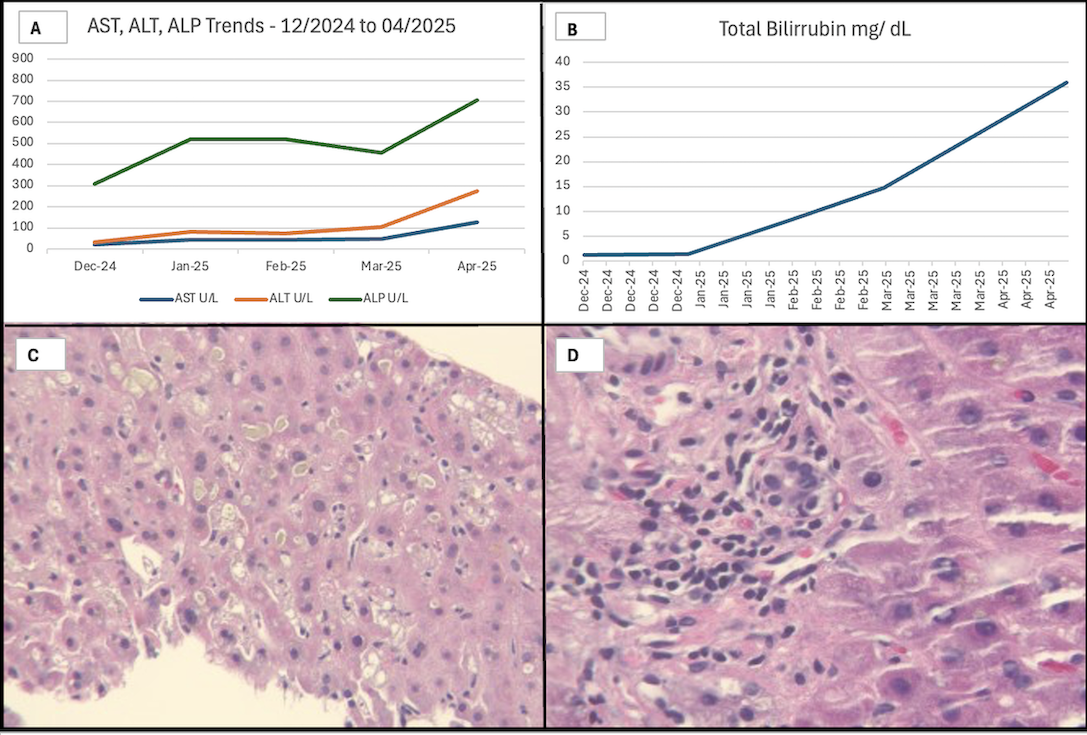
Figure: Figure 1: 1A. AST, ALT, and Alkaline Phosphatase (ALP) trends. The liver enzymes exceeded 1.5 x the normal range, as described in the literature. 1 B. Total bilirubin range (usually reported as high as 20 mg/dL in the literature). 1C. An H&E stain of the liver biopsy showed lobular cholestasis. 1D. An H&E stain of the liver biopsy showed ductulitis/ ductular reaction of cholangiocytes.
Disclosures:
Aryanna Sousa indicated no relevant financial relationships.
Giovannie Isaac-Coss indicated no relevant financial relationships.
Edie Chan indicated no relevant financial relationships.
Lloyd Brown indicated no relevant financial relationships.
Nathalie Sela indicated no relevant financial relationships.
John Klein indicated no relevant financial relationships.
Justin Mitchell indicated no relevant financial relationships.
Nancy Reau: AbbVie – Grant/Research Support. Arbutus – Advisor or Review Panel Member. Gilead – Advisory Committee/Board Member, Grant/Research Support. Salix – Advisory Committee/Board Member, Grant/Research Support. VIR – Advisory Committee/Board Member, Grant/Research Support.
Aryanna Sousa, MD, MPH1, Giovannie Isaac-Coss, MD2, Edie Chan, MD2, Lloyd Brown, MD2, Nathalie Sela, MD2, John Klein, MD2, Justin Mitchell, MD2, Nancy Reau, MD, FACG2. P6119 - First Fatal Case Report of Post-COVID-19 Cholangiopathy in a Liver Transplant Recipient, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rush University Medical Center, Providence, RI; 2Rush University Medical Center, Chicago, IL
Introduction: Post-COVID cholangiopathy (PCC) is a rare variant of secondary sclerosing cholangitis, typically arising after severe COVID-19, particularly in ICU patients or those requiring prolonged ventilation. It is marked by rapid biliary injury, dramatic elevations in ALP and bilirubin, and can progress to liver failure. PCC affects < 0.1% of hospitalized COVID-19 patients and carries a 30–50% mortality. In liver transplant recipients, it can lead to significant graft dysfunction or loss.
Case Description/
Methods: We report a case of a 67-year-old woman with type 2 diabetes, COPD, emphysema, and alpha-1 antitrypsin deficiency-related cirrhosis who underwent liver transplantation. She was maintained on cyclosporin due to tacrolimus-induced neurotoxicity and experienced post-transplant biliary strictures managed with ERCPs and stents, for which she received Augmentin for prophylaxis at least 3 times.
Her bilirubin remained ≤1.9 mg/dL until post-op month 10. At month 11, she contracted COVID-19, for which she was previously vaccinated. Symptoms were mild, and she recovered at home without treatment. Meanwhile, she also completed a two-week course of Augmentin for otitis media. By month 12, on routine lab check, bilirubin sharply rose to 8.4 mg/dL, with GGT persistently ranging at 200–300 U/L. Repeat ERCPs showed no new strictures. Bilirubin continued to rise. Ursodiol was initiated, and immunosuppression was switched back to tacrolimus, though levels remained therapeutic and there was no clinical improvement.
At post-op month 13, she was admitted with acute liver failure and shock. Liver biopsy revealed mild rejection, severe cholestasis, and focal lymphocytic cholangitis, without signs of drug-induced liver injury (DILI). Due to frailty, she was not re-listed for transplant. She died four days after being transferred for a second opinion. Final labs: bilirubin 55.3 mg/dL, ALP 293 U/L, AST 69 U/L, ALT 43 U/L, INR 1.6.
Discussion: This is the first reported case of PCC in a liver transplant recipient. Uniquely, the patient developed PCC after a mild COVID-19 illness. Chronic immunosuppression may have contributed to the severity and fatality. SARS-CoV-2 directly injures cholangiocytes via ACE2 receptors. Although DILI was not evident on biopsy, repeated Augmentin use may have played a role. Given its high mortality, early recognition of PCC in transplant patients is critical to consider re-listing before irreversible graft loss.

Figure: Figure 1: 1A. AST, ALT, and Alkaline Phosphatase (ALP) trends. The liver enzymes exceeded 1.5 x the normal range, as described in the literature. 1 B. Total bilirubin range (usually reported as high as 20 mg/dL in the literature). 1C. An H&E stain of the liver biopsy showed lobular cholestasis. 1D. An H&E stain of the liver biopsy showed ductulitis/ ductular reaction of cholangiocytes.
Disclosures:
Aryanna Sousa indicated no relevant financial relationships.
Giovannie Isaac-Coss indicated no relevant financial relationships.
Edie Chan indicated no relevant financial relationships.
Lloyd Brown indicated no relevant financial relationships.
Nathalie Sela indicated no relevant financial relationships.
John Klein indicated no relevant financial relationships.
Justin Mitchell indicated no relevant financial relationships.
Nancy Reau: AbbVie – Grant/Research Support. Arbutus – Advisor or Review Panel Member. Gilead – Advisory Committee/Board Member, Grant/Research Support. Salix – Advisory Committee/Board Member, Grant/Research Support. VIR – Advisory Committee/Board Member, Grant/Research Support.
Aryanna Sousa, MD, MPH1, Giovannie Isaac-Coss, MD2, Edie Chan, MD2, Lloyd Brown, MD2, Nathalie Sela, MD2, John Klein, MD2, Justin Mitchell, MD2, Nancy Reau, MD, FACG2. P6119 - First Fatal Case Report of Post-COVID-19 Cholangiopathy in a Liver Transplant Recipient, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

