Tuesday Poster Session
Category: Liver
A Suspected Case of Drug Induced Liver Injury Due to Tongkat Ali (<i>Eurycoma longifolia</i>)
P6117 - A Suspected Case of Drug Induced Liver Injury Due to Tongkat Ali (Eurycoma longifolia)
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bhagyashree Behera, BS (she/her/hers)
University of North Carolina at Chapel Hill
Chapel Hill, NC
Presenting Author(s)
Bhagyashree Behera, BS1, Alfred Barritt, MD, MSCR, FACG2
1University of North Carolina at Chapel Hill, Chapel Hill, NC; 2University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Introduction: Herbal and dietary supplements (HDS) are used with increasing frequency for numerous reasons without clear proven efficacy or safety. The root extract of tongkat ali (Eurycoma longifolia) commonly known as “Long Jack”, has been used to treat erectile dysfunction and increase free testosterone. We present a rare case of a suspected hepatocellular drug induced liver injury (DILI) due to tongkat ali.
Case Description/
Methods: 47-year-old healthy male with no prior history of liver disease or substance use disorder presented with elevated liver enzymes in October 2023 [TB 1.1 mg/dL, AST 78 U/L, ALT 165 U/L, AP 59 U/L]. The patient had been taking numerous HDS products since April 2023: resveratrol, niagen (NAD+), L-Tyrosine, creatine, magnesium L-Threonate, Vitamin D3, Vitamin K, inositol, glycine, and tongkat ali (Table 1). The patient was using HDS to increase testosterone and support muscle growth.
The patient had persistently elevated ALT values (peak 236 U/L) on repeat labs in November 2023 but was negative for viral & autoimmune hepatitis and had normal liver imaging. Vibration controlled transient elastography showed no fibrosis and grade 3 steatosis. The patient had no signs or symptoms of liver failure. The patient was instructed to stop all HDS intake and ALT level decreased to < 100 U/L in January 2024, eventually normalizing by April 2024 [Figure 1A]. The patient is suspected of having experienced a hepatocellular DILI (R value =6.4), most likely attributed to tongkat ali.
Discussion: Though a DILI due to tongkat ali is rare, it has been reported to manifest in a mild hepatocellular injury pattern as in this case. Identifying the causal agent in HDS DILI is often challenging as patients frequently take multiple supplements at the same time and/or may take these products inconsistently without clear starting and stopping dates. The patient used tongkat ali intermittently, which may explain the injury after months of use under a challenge/re-challenge paradigm. Additionally, his prevalent steatosis may have predisposed him to liver injury. The other HDS the patient consumed were unlikely to cause a DILI (Table 1). The patient reintroduced creatine and magnesium L-Threonate supplements after January 2024 but has maintained normal liver enzymes. Providers should be aware that tongkat ali is growing in popularity due to its marketed testosterone promoting benefits. Patients interested in using tongkat ali should be educated on the potential risks associated with this product.
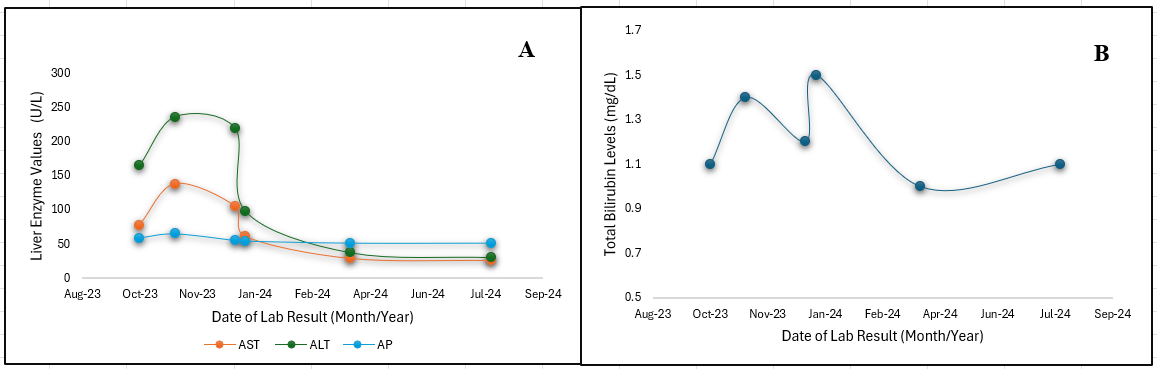
Figure: Table 1: Comprehensive summary of the patient’s herbal and dietary supplements. aLiverTox likelihood of DILI score: D = possible rare cause, <3 reported cases ; E = unlikely, no evidence or reports; X= unknown.
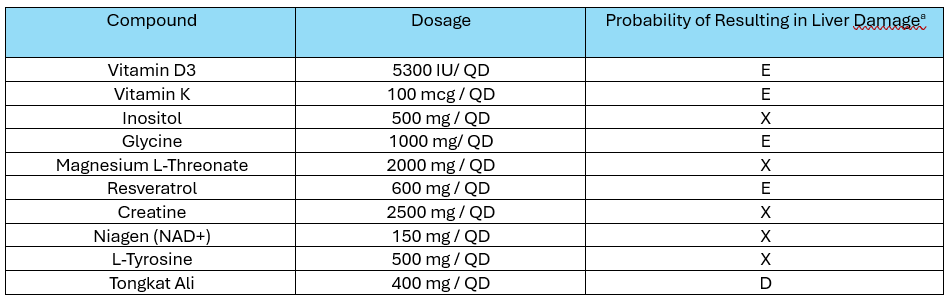
Figure: Figure 1. A: Graph demonstrating the downward trend of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (AP) over time. (B): Graph demonstrating the downward trend of total bilirubin levels (mg/dL) over time.
Disclosures:
Bhagyashree Behera indicated no relevant financial relationships.
Alfred Barritt: Boehringer Ingleheim – Consultant. Madrigal – Consultant. Merck – Consultant. Target RWE – Consultant.
Bhagyashree Behera, BS1, Alfred Barritt, MD, MSCR, FACG2. P6117 - A Suspected Case of Drug Induced Liver Injury Due to Tongkat Ali (<i>Eurycoma longifolia</i>), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of North Carolina at Chapel Hill, Chapel Hill, NC; 2University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Introduction: Herbal and dietary supplements (HDS) are used with increasing frequency for numerous reasons without clear proven efficacy or safety. The root extract of tongkat ali (Eurycoma longifolia) commonly known as “Long Jack”, has been used to treat erectile dysfunction and increase free testosterone. We present a rare case of a suspected hepatocellular drug induced liver injury (DILI) due to tongkat ali.
Case Description/
Methods: 47-year-old healthy male with no prior history of liver disease or substance use disorder presented with elevated liver enzymes in October 2023 [TB 1.1 mg/dL, AST 78 U/L, ALT 165 U/L, AP 59 U/L]. The patient had been taking numerous HDS products since April 2023: resveratrol, niagen (NAD+), L-Tyrosine, creatine, magnesium L-Threonate, Vitamin D3, Vitamin K, inositol, glycine, and tongkat ali (Table 1). The patient was using HDS to increase testosterone and support muscle growth.
The patient had persistently elevated ALT values (peak 236 U/L) on repeat labs in November 2023 but was negative for viral & autoimmune hepatitis and had normal liver imaging. Vibration controlled transient elastography showed no fibrosis and grade 3 steatosis. The patient had no signs or symptoms of liver failure. The patient was instructed to stop all HDS intake and ALT level decreased to < 100 U/L in January 2024, eventually normalizing by April 2024 [Figure 1A]. The patient is suspected of having experienced a hepatocellular DILI (R value =6.4), most likely attributed to tongkat ali.
Discussion: Though a DILI due to tongkat ali is rare, it has been reported to manifest in a mild hepatocellular injury pattern as in this case. Identifying the causal agent in HDS DILI is often challenging as patients frequently take multiple supplements at the same time and/or may take these products inconsistently without clear starting and stopping dates. The patient used tongkat ali intermittently, which may explain the injury after months of use under a challenge/re-challenge paradigm. Additionally, his prevalent steatosis may have predisposed him to liver injury. The other HDS the patient consumed were unlikely to cause a DILI (Table 1). The patient reintroduced creatine and magnesium L-Threonate supplements after January 2024 but has maintained normal liver enzymes. Providers should be aware that tongkat ali is growing in popularity due to its marketed testosterone promoting benefits. Patients interested in using tongkat ali should be educated on the potential risks associated with this product.

Figure: Table 1: Comprehensive summary of the patient’s herbal and dietary supplements. aLiverTox likelihood of DILI score: D = possible rare cause, <3 reported cases ; E = unlikely, no evidence or reports; X= unknown.

Figure: Figure 1. A: Graph demonstrating the downward trend of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (AP) over time. (B): Graph demonstrating the downward trend of total bilirubin levels (mg/dL) over time.
Disclosures:
Bhagyashree Behera indicated no relevant financial relationships.
Alfred Barritt: Boehringer Ingleheim – Consultant. Madrigal – Consultant. Merck – Consultant. Target RWE – Consultant.
Bhagyashree Behera, BS1, Alfred Barritt, MD, MSCR, FACG2. P6117 - A Suspected Case of Drug Induced Liver Injury Due to Tongkat Ali (<i>Eurycoma longifolia</i>), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
