Tuesday Poster Session
Category: Liver
P6113 - Testosterone-Induced Drug-Induced Liver Injury Progressing to Acute Liver Failure: A Case Report on a Non-FDA-Regulated Testosterone Pellet
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ishan Antony, MD
Beth Israel Lahey Health
Burlington, MA
Presenting Author(s)
Ishan Antony, MD1, Ali Shaat, MD2, Yougen Zhan, MD2, Taha Shakarchi, MD2, Ming V.. Lin, MD2
1Beth Israel Lahey Health, Burlington, MA; 2Lahey Hospital and Medical Center, Burlington, MA
Introduction: Testosterone is used for hypogonadism and could be misused for androgenic effects. Hepatotoxicity is rare with FDA-approved forms, mainly linked to oral alkylated agents. We present a case of severe cholestatic Drug-Induced Liver Injury (DILI) secondary to compounded testosterone pellets (cTP), ultimately progressing to acute liver failure (ALF) and multi-organ failure.
Case Description/
Methods: A 65-year-old male with hyperlipidemia presented with fatigue, nausea, jaundice, and pruritus two weeks after re-implantation of a Biote cTP, which he used for seven years. He was alert, icteric, without other signs of chronic liver disease. Labs: AST 200, ALT 366, ALP 687, total bili (TB) 15.5, albumin 2.2, Na 122, INR 1.7, Cr 2.81. Viral and autoimmune hepatitis workup was negative. MRCP showed a normal liver and biliary tree. Liver biopsy revealed moderate to severe lobular cholestasis without necrosis, consistent with DILI. Despite ursodiol, rifaximin, and lactulose, he developed worsening encephalopathy and cholestasis (TB 45.2), and renal failure (Cr 4.37). Eight cTPs were surgically removed from the right gluteus. He underwent transplant evaluation; cardiac cath showed 80% LAD stenosis requiring a Drug-Eluting Stent. Discharged on aspirin and clopidogrel. Two weeks later, he was readmitted with hematemesis and shock. He had severe coagulopathy (INR 5.2, PTT 79), renal failure (Cr 13.4 mg/dL), and hyperbilirubinemia (52.2 mg/dL). EGD showed old blood content in the esophagus and stomach, and diffuse mildly friable mucosa. He developed worsening encephalopathy and died of cardiovascular collapse on day 49 despite intensive care.
Discussion: This case highlights severe cholestatic DILI progressing to ALF following non-FDA cTP exposure. cTPs are subcutaneously implanted for steady hormone release but lack safety oversight and may cause DILI. The biopsy finding of bland cholestasis was suggestive of androgenic DILI from cTP, likely causing ALF. Progression to ALF is exceedingly rare in testosterone-induced DILI, with no prior reports linked to cTP. While the patient had tolerated cTP for several years, this episode likely reflects an idiosyncratic reaction precipitated by cumulative exposure, a potentially supratherapeutic compounded dose, or impaired hepatic resilience due to recent dehydration, NSAID use, or infection. The latency between drug exposure and injury is consistent with reported cases of androgenic DILI. The case underscores the hepatotoxic risk of cTP and the need for oversight.
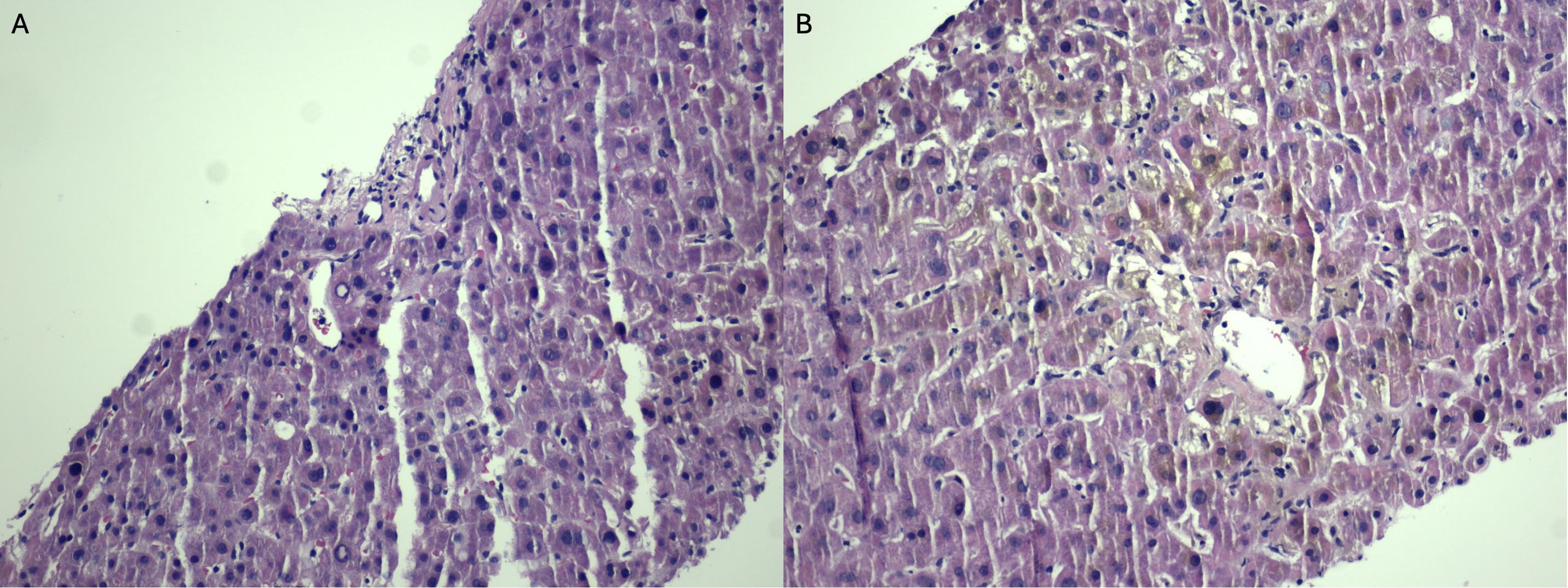
Figure: Figure 1: (A) Low-power H&E stain showing a bland portal tract and lobular architecture, without significant inflammation, ductal injury, or interface activity consistent with bland cholestasis. (B) High-power view revealing canalicular bile plugs and feathery hepatocyte degeneration, characteristic of cholestatic injury seen in androgenic steroid-induced DILI.

Figure: Table 1: Laboratory trends demonstrating progressive cholestasis, hepatic dysfunction, and renal failure over the hospital course.
Disclosures:
Ishan Antony indicated no relevant financial relationships.
Ali Shaat indicated no relevant financial relationships.
Yougen Zhan indicated no relevant financial relationships.
Taha Shakarchi indicated no relevant financial relationships.
Ming Lin indicated no relevant financial relationships.
Ishan Antony, MD1, Ali Shaat, MD2, Yougen Zhan, MD2, Taha Shakarchi, MD2, Ming V.. Lin, MD2. P6113 - Testosterone-Induced Drug-Induced Liver Injury Progressing to Acute Liver Failure: A Case Report on a Non-FDA-Regulated Testosterone Pellet, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Beth Israel Lahey Health, Burlington, MA; 2Lahey Hospital and Medical Center, Burlington, MA
Introduction: Testosterone is used for hypogonadism and could be misused for androgenic effects. Hepatotoxicity is rare with FDA-approved forms, mainly linked to oral alkylated agents. We present a case of severe cholestatic Drug-Induced Liver Injury (DILI) secondary to compounded testosterone pellets (cTP), ultimately progressing to acute liver failure (ALF) and multi-organ failure.
Case Description/
Methods: A 65-year-old male with hyperlipidemia presented with fatigue, nausea, jaundice, and pruritus two weeks after re-implantation of a Biote cTP, which he used for seven years. He was alert, icteric, without other signs of chronic liver disease. Labs: AST 200, ALT 366, ALP 687, total bili (TB) 15.5, albumin 2.2, Na 122, INR 1.7, Cr 2.81. Viral and autoimmune hepatitis workup was negative. MRCP showed a normal liver and biliary tree. Liver biopsy revealed moderate to severe lobular cholestasis without necrosis, consistent with DILI. Despite ursodiol, rifaximin, and lactulose, he developed worsening encephalopathy and cholestasis (TB 45.2), and renal failure (Cr 4.37). Eight cTPs were surgically removed from the right gluteus. He underwent transplant evaluation; cardiac cath showed 80% LAD stenosis requiring a Drug-Eluting Stent. Discharged on aspirin and clopidogrel. Two weeks later, he was readmitted with hematemesis and shock. He had severe coagulopathy (INR 5.2, PTT 79), renal failure (Cr 13.4 mg/dL), and hyperbilirubinemia (52.2 mg/dL). EGD showed old blood content in the esophagus and stomach, and diffuse mildly friable mucosa. He developed worsening encephalopathy and died of cardiovascular collapse on day 49 despite intensive care.
Discussion: This case highlights severe cholestatic DILI progressing to ALF following non-FDA cTP exposure. cTPs are subcutaneously implanted for steady hormone release but lack safety oversight and may cause DILI. The biopsy finding of bland cholestasis was suggestive of androgenic DILI from cTP, likely causing ALF. Progression to ALF is exceedingly rare in testosterone-induced DILI, with no prior reports linked to cTP. While the patient had tolerated cTP for several years, this episode likely reflects an idiosyncratic reaction precipitated by cumulative exposure, a potentially supratherapeutic compounded dose, or impaired hepatic resilience due to recent dehydration, NSAID use, or infection. The latency between drug exposure and injury is consistent with reported cases of androgenic DILI. The case underscores the hepatotoxic risk of cTP and the need for oversight.

Figure: Figure 1: (A) Low-power H&E stain showing a bland portal tract and lobular architecture, without significant inflammation, ductal injury, or interface activity consistent with bland cholestasis. (B) High-power view revealing canalicular bile plugs and feathery hepatocyte degeneration, characteristic of cholestatic injury seen in androgenic steroid-induced DILI.

Figure: Table 1: Laboratory trends demonstrating progressive cholestasis, hepatic dysfunction, and renal failure over the hospital course.
Disclosures:
Ishan Antony indicated no relevant financial relationships.
Ali Shaat indicated no relevant financial relationships.
Yougen Zhan indicated no relevant financial relationships.
Taha Shakarchi indicated no relevant financial relationships.
Ming Lin indicated no relevant financial relationships.
Ishan Antony, MD1, Ali Shaat, MD2, Yougen Zhan, MD2, Taha Shakarchi, MD2, Ming V.. Lin, MD2. P6113 - Testosterone-Induced Drug-Induced Liver Injury Progressing to Acute Liver Failure: A Case Report on a Non-FDA-Regulated Testosterone Pellet, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
