Tuesday Poster Session
Category: Practice Management
P6182 - Ten-Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent United States Food and Drug Administration Approval of Therapeutics in Gastroenterology and Hepatology
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ryan F. Hughes, MD
NewYork-Presbyterian / Weill Cornell Medical Center
New York, NY
Presenting Author(s)
Ryan F. Hughes, MD1, David Li, BS2, Xiaohan Ying, MD1, Russell Rosenblatt, MD, MS1, Arun Jesudian, MD, FACG3
1NewYork-Presbyterian / Weill Cornell Medical Center, New York, NY; 2Weill Cornell College of Cornell University, New York, NY; 3NewYork-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY
Introduction: In November 2020, the United States Food and Drug Administration (FDA) released updated guidance to increase the clinical trial enrollment of underrepresented populations, including women and racial/ethnic minorities, to ensure the widespread safety and efficacy of novel therapeutics. This study aims to investigate the 10-year trends in participation of women and racial/ethnic minorities in pivotal trials supporting the approval of new gastrointestinal/hepatobiliary drugs from January 2015 to December 2024.
Methods: A list of novel pharmacologic agents approved for conditions within gastroenterology/hepatology was abstracted from publicly available data from Drugs@FDA. Publicly available sex and race/ethnicity data were collected from the individual trials via ClinicalTrials.gov. Linear regression analysis was performed to assess the relation between drug approval year and proportion of women and minorities enrolled.
Results: Forty-one (n=41) novel therapeutics for gastrointestinal/hepatobiliary conditions were approved by the FDA throughout the study period. The median number of participants supporting each drug was 691 (interquartile range, 376–2158). Women represented 57% (n=36,141) of trial participants (n=63,242). Males represented the majority in drug trials after 2020 (up to 62% in 2024). There was no significant association between enrollment of women with drug approval year (p=0.298). Among trial participants, 56% were White, 6% Black, 9% Asian, 7% Hispanic/Latino. There was no significant association between White (p=0.351), Black (p=0.697), or Hispanic/Latino (p=0.927) enrollment with drug approval year. However, the proportion of Asian enrollment increased over time (β = 0.032, 95% CI 0.007–0.057, p = 0.016).
Discussion: From 2015-2024, there has been stable representation of females in clinical trial investigations for novel therapeutic agents within gastroenterology/hepatology. Despite 2020 FDA guidance efforts to increase female representation in clinical trials, males represented the majority from 2021–2024. There was a significant upward trend in Asian enrollment over time. Despite accounting for 14% and 19% of the total U.S. population, Black and Hispanic/Latino patients only accounted for 6% and 7% of the clinical trials population. Gender and racial/ethnicity equity in the participation of clinical trials is fundamental to the assurance of widespread safety and efficacy of novel therapeutics, highlighting the need for continued monitoring of future enrollment trends.
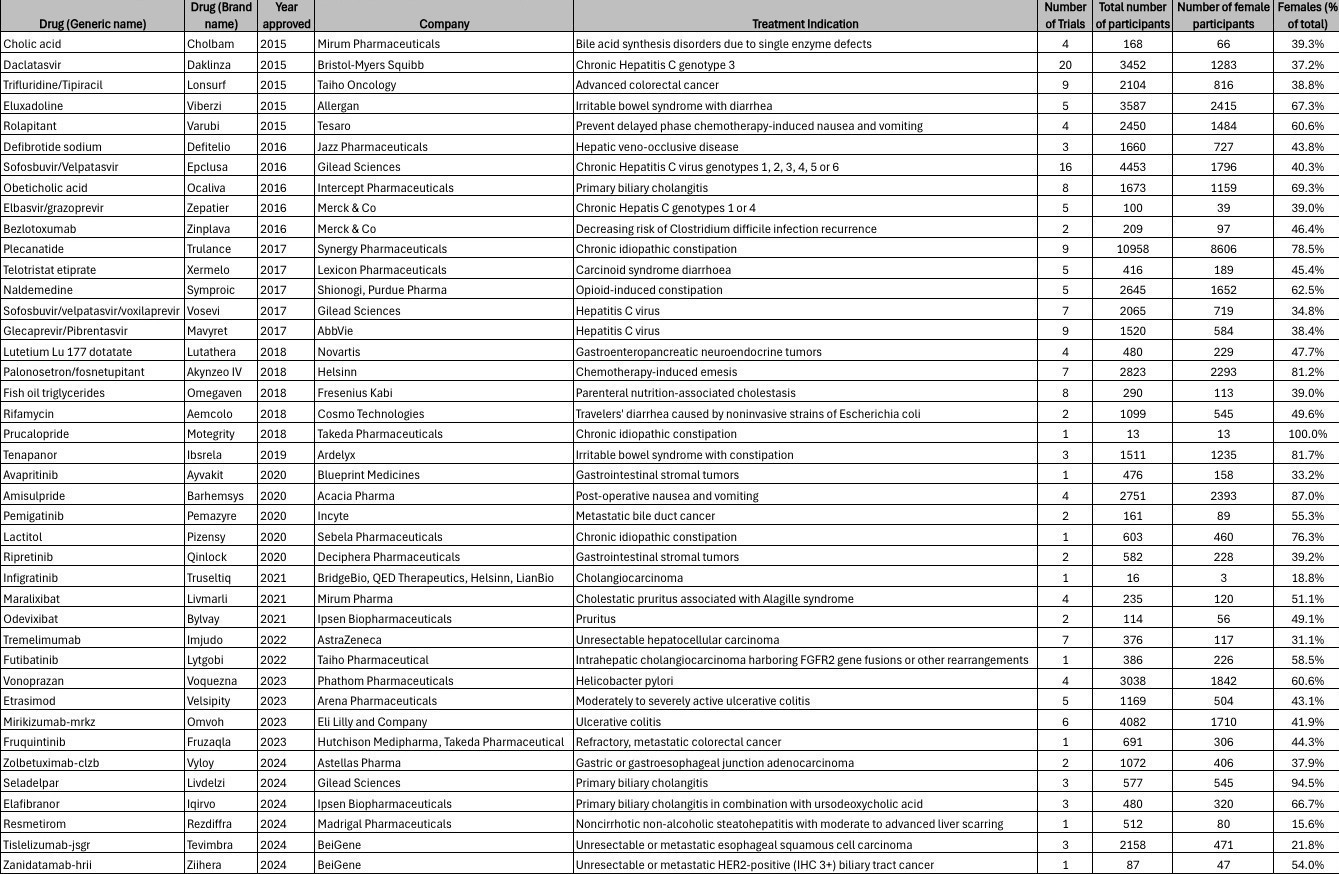
Figure: Figure 1. Characteristics of novel gastrointestinal/hepatobiliary drugs approved in the past decade.
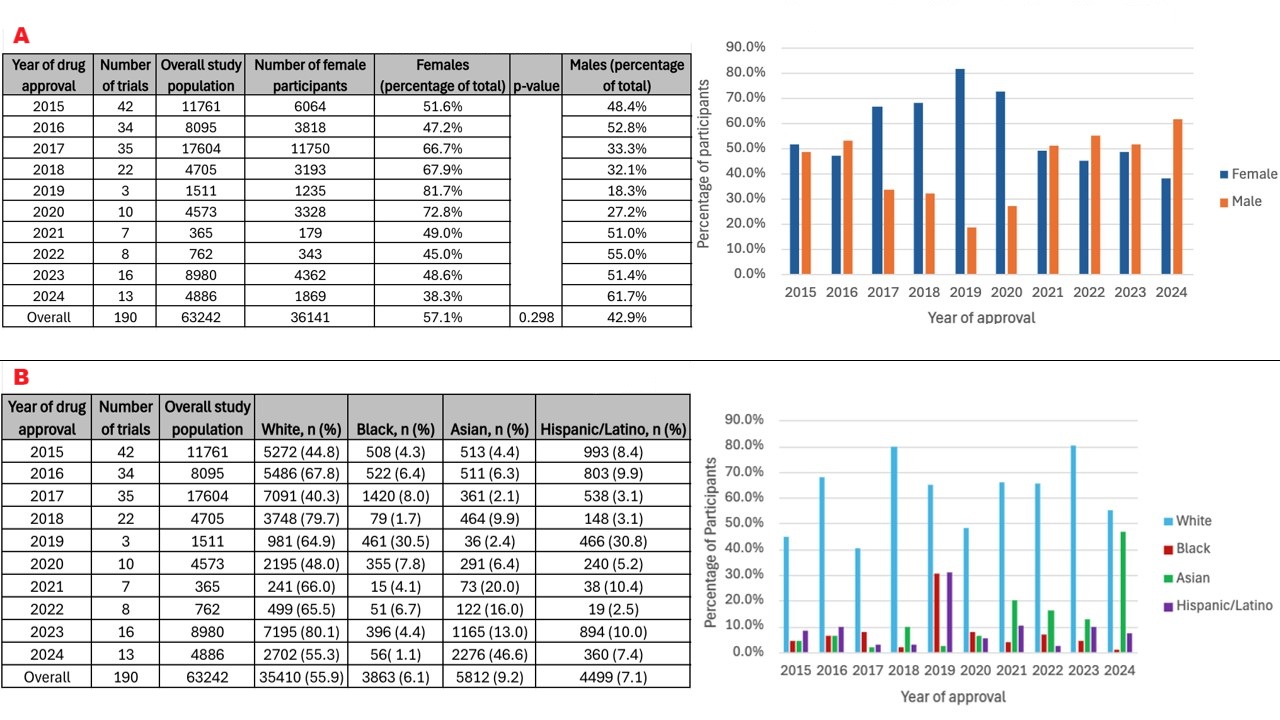
Figure: Figure 2. Representation of (A) women and (B) ethnic/racial minorities in pivotal gastrointestinal/hepatobiliary drug trials from 2015-2024 in both chart and graphical formats. Note: Some trials reported Hispanic/Latino patients under “Race,” while others reported under “Ethnicity.” Additionally, some trials further delineated “White Hispanic” versus “Black Hispanic” as patient identifiers, however the majority of the trials did not specify. Therefore, there is some overlap in the reported race/ethnicity data, as it pertains to patients identifying as both Hispanic/Latino and White or Black, concurrently. Patients classified as “other” or “unknown/not reported” are not displayed.
Disclosures:
Ryan Hughes indicated no relevant financial relationships.
David Li indicated no relevant financial relationships.
Xiaohan Ying indicated no relevant financial relationships.
Russell Rosenblatt indicated no relevant financial relationships.
Arun Jesudian: Madrigal Pharmaceuticals – Speakers Bureau. Mallinckrodt Pharmaceuticals – Consultant, Speakers Bureau. Salix Pharmaceuticals – Consultant, Speakers Bureau.
Ryan F. Hughes, MD1, David Li, BS2, Xiaohan Ying, MD1, Russell Rosenblatt, MD, MS1, Arun Jesudian, MD, FACG3. P6182 - Ten-Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent United States Food and Drug Administration Approval of Therapeutics in Gastroenterology and Hepatology, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NewYork-Presbyterian / Weill Cornell Medical Center, New York, NY; 2Weill Cornell College of Cornell University, New York, NY; 3NewYork-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY
Introduction: In November 2020, the United States Food and Drug Administration (FDA) released updated guidance to increase the clinical trial enrollment of underrepresented populations, including women and racial/ethnic minorities, to ensure the widespread safety and efficacy of novel therapeutics. This study aims to investigate the 10-year trends in participation of women and racial/ethnic minorities in pivotal trials supporting the approval of new gastrointestinal/hepatobiliary drugs from January 2015 to December 2024.
Methods: A list of novel pharmacologic agents approved for conditions within gastroenterology/hepatology was abstracted from publicly available data from Drugs@FDA. Publicly available sex and race/ethnicity data were collected from the individual trials via ClinicalTrials.gov. Linear regression analysis was performed to assess the relation between drug approval year and proportion of women and minorities enrolled.
Results: Forty-one (n=41) novel therapeutics for gastrointestinal/hepatobiliary conditions were approved by the FDA throughout the study period. The median number of participants supporting each drug was 691 (interquartile range, 376–2158). Women represented 57% (n=36,141) of trial participants (n=63,242). Males represented the majority in drug trials after 2020 (up to 62% in 2024). There was no significant association between enrollment of women with drug approval year (p=0.298). Among trial participants, 56% were White, 6% Black, 9% Asian, 7% Hispanic/Latino. There was no significant association between White (p=0.351), Black (p=0.697), or Hispanic/Latino (p=0.927) enrollment with drug approval year. However, the proportion of Asian enrollment increased over time (β = 0.032, 95% CI 0.007–0.057, p = 0.016).
Discussion: From 2015-2024, there has been stable representation of females in clinical trial investigations for novel therapeutic agents within gastroenterology/hepatology. Despite 2020 FDA guidance efforts to increase female representation in clinical trials, males represented the majority from 2021–2024. There was a significant upward trend in Asian enrollment over time. Despite accounting for 14% and 19% of the total U.S. population, Black and Hispanic/Latino patients only accounted for 6% and 7% of the clinical trials population. Gender and racial/ethnicity equity in the participation of clinical trials is fundamental to the assurance of widespread safety and efficacy of novel therapeutics, highlighting the need for continued monitoring of future enrollment trends.

Figure: Figure 1. Characteristics of novel gastrointestinal/hepatobiliary drugs approved in the past decade.

Figure: Figure 2. Representation of (A) women and (B) ethnic/racial minorities in pivotal gastrointestinal/hepatobiliary drug trials from 2015-2024 in both chart and graphical formats. Note: Some trials reported Hispanic/Latino patients under “Race,” while others reported under “Ethnicity.” Additionally, some trials further delineated “White Hispanic” versus “Black Hispanic” as patient identifiers, however the majority of the trials did not specify. Therefore, there is some overlap in the reported race/ethnicity data, as it pertains to patients identifying as both Hispanic/Latino and White or Black, concurrently. Patients classified as “other” or “unknown/not reported” are not displayed.
Disclosures:
Ryan Hughes indicated no relevant financial relationships.
David Li indicated no relevant financial relationships.
Xiaohan Ying indicated no relevant financial relationships.
Russell Rosenblatt indicated no relevant financial relationships.
Arun Jesudian: Madrigal Pharmaceuticals – Speakers Bureau. Mallinckrodt Pharmaceuticals – Consultant, Speakers Bureau. Salix Pharmaceuticals – Consultant, Speakers Bureau.
Ryan F. Hughes, MD1, David Li, BS2, Xiaohan Ying, MD1, Russell Rosenblatt, MD, MS1, Arun Jesudian, MD, FACG3. P6182 - Ten-Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent United States Food and Drug Administration Approval of Therapeutics in Gastroenterology and Hepatology, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

