Tuesday Poster Session
Category: Stomach and Spleen
P6333 - PP‑Why? Evaluation of Proton Pump Inhibitor Prescribing Practices in a Primary Care Clinic
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Abrahim Mahmood, DO (he/him/his)
NewYork-Presbyterian/Queens
Greenlawn, NY
Presenting Author(s)
Abrahim Mahmood, DO1, Kathleen Horan, PharmD2, Reena Razdan, MD2, Bhojranie Brahmanand, 2, Lauren Anderson, PharmD2, Blanca Sckell, MD, MPH2, Constantine Fisher, MD2
1NewYork-Presbyterian/Queens, Greenlawn, NY; 2NewYork-Presbyterian/Queens, Flushing, NY
Introduction: Proton pump inhibitors (PPIs) are recommended for short‐term management of acid‐mediated upper gastrointestinal conditions but are often continued for poorly defined indications. Long‑term PPI use has been linked to enteric infections, chronic kidney disease, fractures, and dementia. One ambulatory care center estimated inappropriate PPI prescribing costs at $1.5 million annually, and up to two‑thirds of users lack a clear indication. National guidelines now advocate structured deprescribing for patients without definitive long‑term indications. To address this issue locally, we sought to evaluate PPI prescribing practices in the NYPQ Primary Care clinic, identify patients eligible for deprescribing, and uncover opportunities for future research, patient care, and education.
Methods: We conducted a retrospective, cross‑sectional review of electronic medical records of adult patients (≥ 18 years) prescribed a PPI for ≥ 30 days and seen October–December 2024 in the NYPQ Primary Care clinic. Deceased and palliative/hospice patients were excluded. Pharmacy and resident teams extracted demographics, PPI indication, and duration. Long‑term use was deemed appropriate for Barrett’s esophagus, LA grade C/D esophagitis, GERD‑related strictures, high ulcer‑bleeding risk on NSAIDs, or recurrent ulcer bleeding prophylaxis. Indications such as refractory functional dyspepsia, steroid use without NSAIDs, non‑ulcer bleeding, and isolated lower GI symptoms were classified as inappropriate. Patients were grouped as eligible for deprescribing, ineligible, or requiring further review.
Results: Of 199 patients meeting inclusion criteria, 21 (10.5%) had discontinued PPIs. Among the remaining 178, 47% were eligible for deprescribing, 44% required further review, and 9% were ineligible.
Discussion: Nearly half of long‑term PPI users in our clinic met deprescribing criteria, mirroring findings from similar ambulatory care center studies nationwide. Given the risks of adverse effects, polypharmacy, and economic burden, interprofessional deprescribing initiatives are warranted. We will pilot a clinic‑wide protocol involving patient education, rebound monitoring, and lifestyle support to optimize therapy and enhance patient safety.
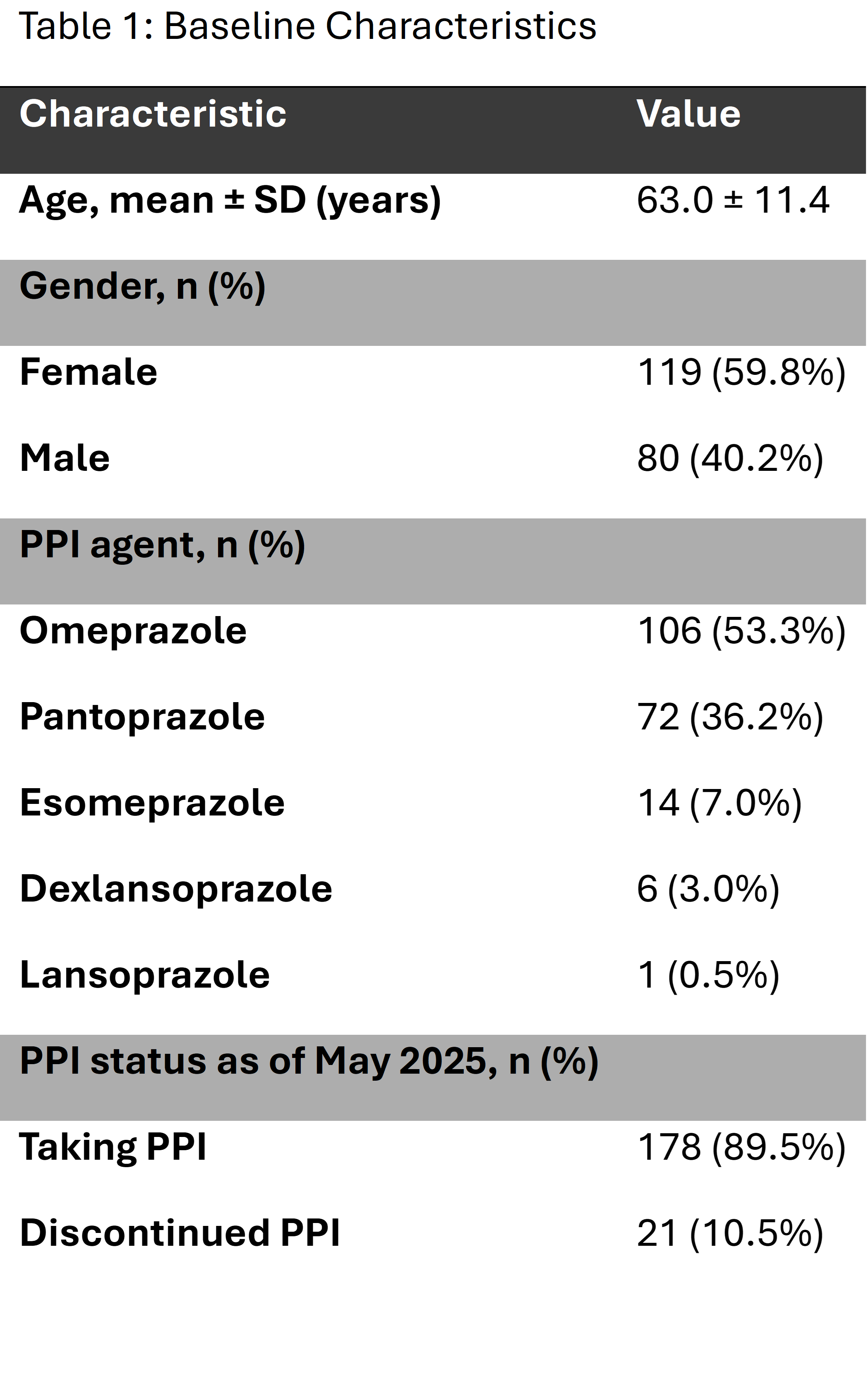
Figure: Figure 1. Flow diagram categorizing 178 chronic PPI users by deprescribing eligibility. Patients with definitive long‑term indications (e.g. Barrett’s esophagus [n = 9], bleeding ulcer history [n = 5], severe esophagitis [n = 2]) were deemed ineligible (n = 16, 9%), while those without clear indications were eligible for deprescribing (n = 84, 47%). The remaining patients (n = 78, 44%) required further chart review to clarify indication.
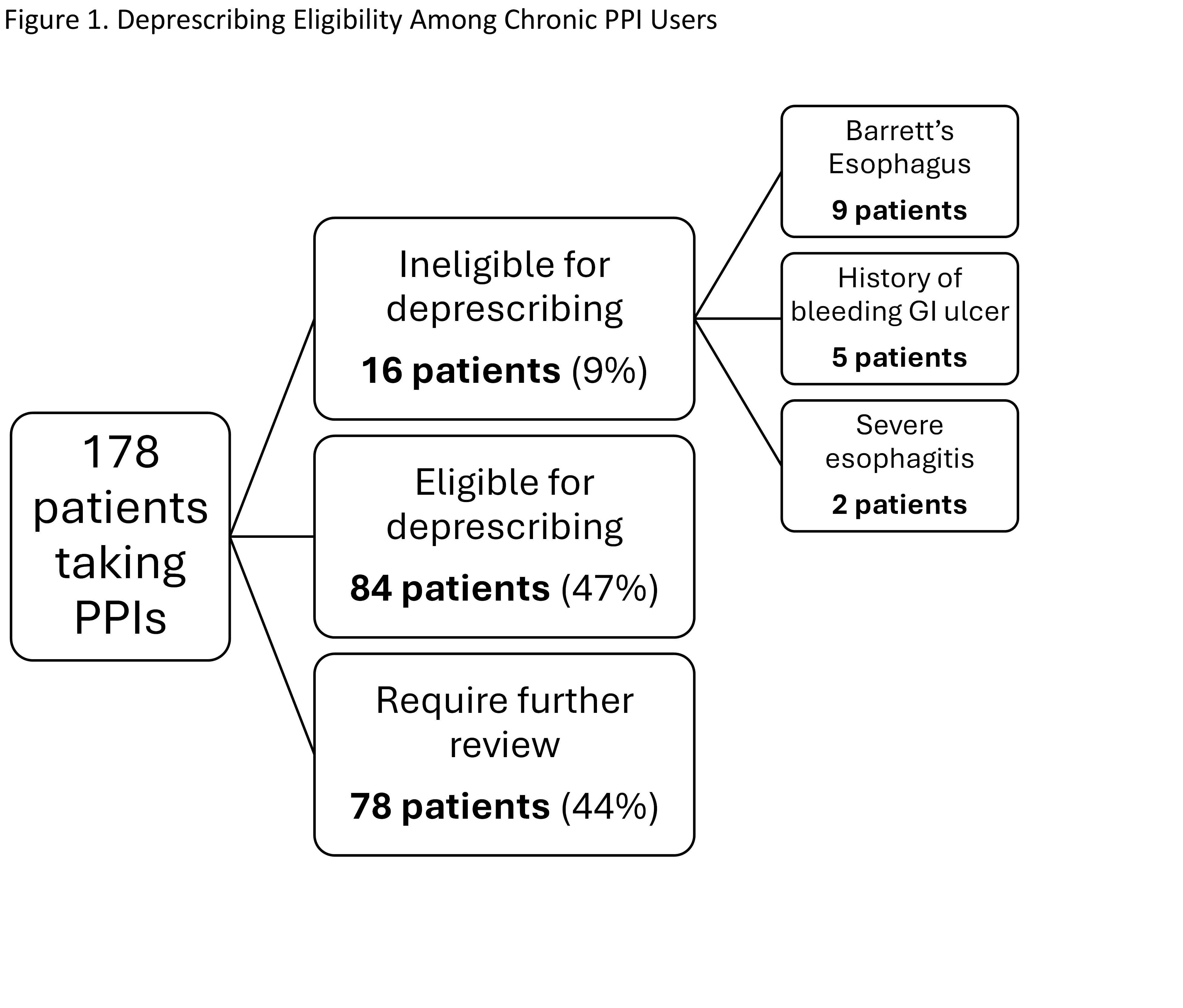
Figure: Table 1. Baseline demographic and clinical characteristics of patients prescribed proton pump inhibitors in the NYPQ Primary Care clinic, including age, gender, PPI agent, and current therapy status.
Disclosures:
Abrahim Mahmood indicated no relevant financial relationships.
Kathleen Horan indicated no relevant financial relationships.
Reena Razdan indicated no relevant financial relationships.
Bhojranie Brahmanand indicated no relevant financial relationships.
Lauren Anderson indicated no relevant financial relationships.
Blanca Sckell indicated no relevant financial relationships.
Constantine Fisher indicated no relevant financial relationships.
Abrahim Mahmood, DO1, Kathleen Horan, PharmD2, Reena Razdan, MD2, Bhojranie Brahmanand, 2, Lauren Anderson, PharmD2, Blanca Sckell, MD, MPH2, Constantine Fisher, MD2. P6333 - PP‑Why? Evaluation of Proton Pump Inhibitor Prescribing Practices in a Primary Care Clinic, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NewYork-Presbyterian/Queens, Greenlawn, NY; 2NewYork-Presbyterian/Queens, Flushing, NY
Introduction: Proton pump inhibitors (PPIs) are recommended for short‐term management of acid‐mediated upper gastrointestinal conditions but are often continued for poorly defined indications. Long‑term PPI use has been linked to enteric infections, chronic kidney disease, fractures, and dementia. One ambulatory care center estimated inappropriate PPI prescribing costs at $1.5 million annually, and up to two‑thirds of users lack a clear indication. National guidelines now advocate structured deprescribing for patients without definitive long‑term indications. To address this issue locally, we sought to evaluate PPI prescribing practices in the NYPQ Primary Care clinic, identify patients eligible for deprescribing, and uncover opportunities for future research, patient care, and education.
Methods: We conducted a retrospective, cross‑sectional review of electronic medical records of adult patients (≥ 18 years) prescribed a PPI for ≥ 30 days and seen October–December 2024 in the NYPQ Primary Care clinic. Deceased and palliative/hospice patients were excluded. Pharmacy and resident teams extracted demographics, PPI indication, and duration. Long‑term use was deemed appropriate for Barrett’s esophagus, LA grade C/D esophagitis, GERD‑related strictures, high ulcer‑bleeding risk on NSAIDs, or recurrent ulcer bleeding prophylaxis. Indications such as refractory functional dyspepsia, steroid use without NSAIDs, non‑ulcer bleeding, and isolated lower GI symptoms were classified as inappropriate. Patients were grouped as eligible for deprescribing, ineligible, or requiring further review.
Results: Of 199 patients meeting inclusion criteria, 21 (10.5%) had discontinued PPIs. Among the remaining 178, 47% were eligible for deprescribing, 44% required further review, and 9% were ineligible.
Discussion: Nearly half of long‑term PPI users in our clinic met deprescribing criteria, mirroring findings from similar ambulatory care center studies nationwide. Given the risks of adverse effects, polypharmacy, and economic burden, interprofessional deprescribing initiatives are warranted. We will pilot a clinic‑wide protocol involving patient education, rebound monitoring, and lifestyle support to optimize therapy and enhance patient safety.

Figure: Figure 1. Flow diagram categorizing 178 chronic PPI users by deprescribing eligibility. Patients with definitive long‑term indications (e.g. Barrett’s esophagus [n = 9], bleeding ulcer history [n = 5], severe esophagitis [n = 2]) were deemed ineligible (n = 16, 9%), while those without clear indications were eligible for deprescribing (n = 84, 47%). The remaining patients (n = 78, 44%) required further chart review to clarify indication.

Figure: Table 1. Baseline demographic and clinical characteristics of patients prescribed proton pump inhibitors in the NYPQ Primary Care clinic, including age, gender, PPI agent, and current therapy status.
Disclosures:
Abrahim Mahmood indicated no relevant financial relationships.
Kathleen Horan indicated no relevant financial relationships.
Reena Razdan indicated no relevant financial relationships.
Bhojranie Brahmanand indicated no relevant financial relationships.
Lauren Anderson indicated no relevant financial relationships.
Blanca Sckell indicated no relevant financial relationships.
Constantine Fisher indicated no relevant financial relationships.
Abrahim Mahmood, DO1, Kathleen Horan, PharmD2, Reena Razdan, MD2, Bhojranie Brahmanand, 2, Lauren Anderson, PharmD2, Blanca Sckell, MD, MPH2, Constantine Fisher, MD2. P6333 - PP‑Why? Evaluation of Proton Pump Inhibitor Prescribing Practices in a Primary Care Clinic, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

