Tuesday Poster Session
Category: Liver
P6099 - A Rash Decision: Navigating Immune-Related Dermatologic and Hepatic Complications in Esophageal Cancer Treatment
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Kavina Munshi, DO (she/her/hers)
Jefferson Torresdale Hospital
Philadelphia, PA
Presenting Author(s)
Kavina Munshi, DO1, Huzaif Taufiq, MD2, Tehilla Apfel, MD3
1Jefferson Torresdale Hospital, Philadelphia, PA; 2Jefferson Torresdale Hospital, Willow Grove, PA; 3Jefferson Health, Langhorne, PA
Introduction: Rituximab, an anti-CD20 monoclonal antibody, treats various autoimmune diseases and has been associated with hepatitis B reactivation. We present a case where a patient, who developed bullous pemphigoid from immunotherapy, was started on rituximab without proper screening, after which his Hepatitis B core antibody came back positive. The patient was subsequently treated for Hepatitis B and completed a course of rituximab.
Case Description/
Methods: A 72-year-old male with history of hypertension, hyperlipidemia, and psoriasis presented with symptoms of worsening dysphagia to his primary care physician’s office. The patient underwent esophagogastroduodenoscopy with findings concerning for distal esophageal adenocarcinoma. He saw Oncology and was treated with radiation, a chemotherapy regimen of carboplatin and paclitaxel and surgical resection. He completed 5 rounds of chemotherapy and started immunotherapy with nivolumab, to be continued for 1 year. He developed a macular rash 7 months into immunotherapy. Thought to be related to his psoriasis, he was treated with topical corticosteroids. However, with no improvement in his symptoms he returned to Oncology and was treated with IVIG. He had mild resolution of his symptoms and was placed on a prednisone taper. He returned a few weeks later with his initial rash. He was referred to Dermatology and started on rituximab. He then had labs drawn with his Gastroenterologist and was positive for hepatitis b core antibody. With concern for Hepatitis B reactivation, he was started on tenofovir alafenamide for suppression. He completed treatment for his bullous pemphigoid and had his hepatic function monitored throughout therapy, which remained normal.
Discussion: This case illustrates a complex interplay between oncologic treatment, immunotherapy-related adverse events, and pre-existing conditions. The initiation of rituximab was aimed at controlling the bullous pemphigoid, which can be triggered by immune checkpoint inhibitors, such as nivolumab. The patient started rituximab without proper screening prior to initiation. This case highlights the importance of vigilance for thorough testing prior to initiating certain monoclonal antibodies, such as rituximab. The patient’s positive hepatitis B core antibody signaled past exposure, and rituximab heightened the risk for reactivation. This case emphasizes the significance of thorough screening, timely recognition, and appropriate prophylactic and therapeutic interventions to optimize patient outcomes.
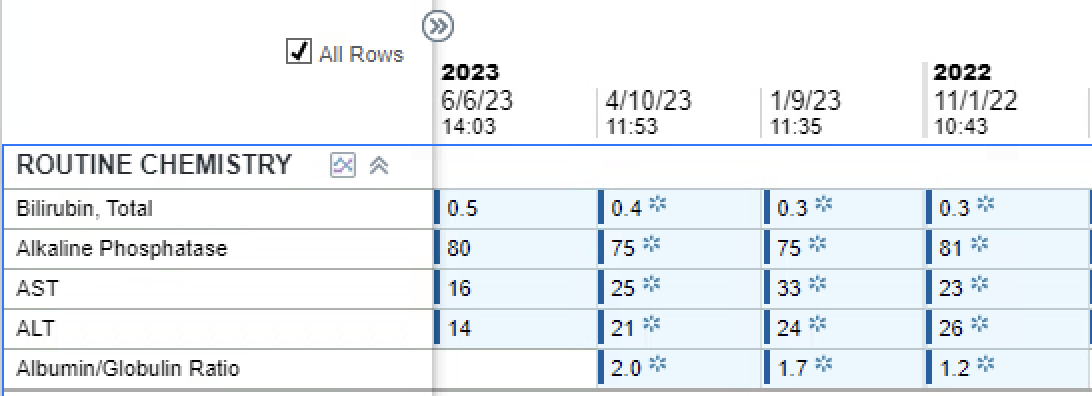
Figure: This table depicts the patient's Hepatitis panel drawn following initiation of rituximab. The Hepatitis B core antibody is positive; however, the remainder of the testing is negative, indicating no acute infection.
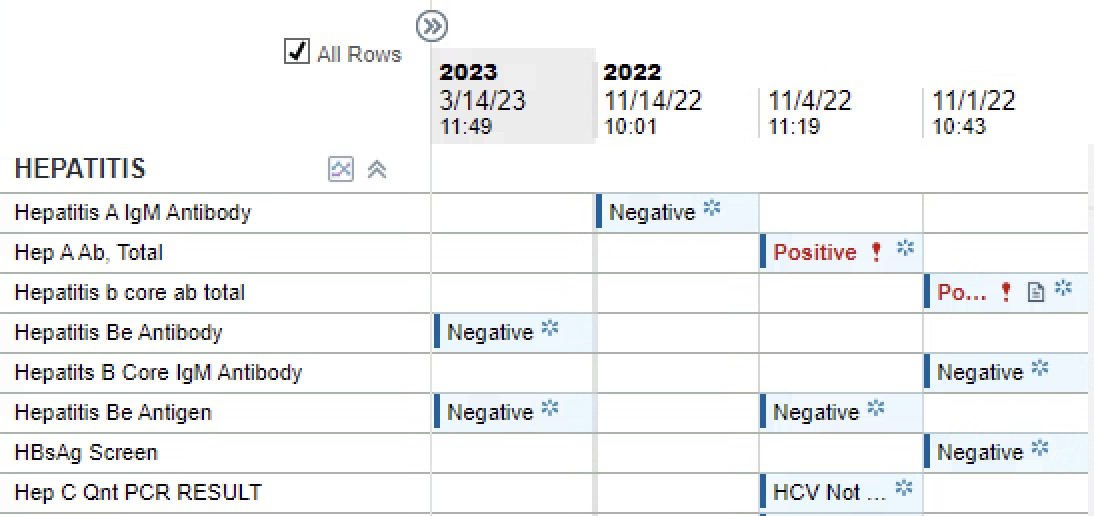
Figure: This table depicts the serial monitoring of the patient's hepatic function while on rituximab and tenofovir alafenamide for adequate suppression therapy. His hepatic function remained normal throughout treatment.
Disclosures:
Kavina Munshi indicated no relevant financial relationships.
Huzaif Taufiq indicated no relevant financial relationships.
Tehilla Apfel indicated no relevant financial relationships.
Kavina Munshi, DO1, Huzaif Taufiq, MD2, Tehilla Apfel, MD3. P6099 - A Rash Decision: Navigating Immune-Related Dermatologic and Hepatic Complications in Esophageal Cancer Treatment, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Jefferson Torresdale Hospital, Philadelphia, PA; 2Jefferson Torresdale Hospital, Willow Grove, PA; 3Jefferson Health, Langhorne, PA
Introduction: Rituximab, an anti-CD20 monoclonal antibody, treats various autoimmune diseases and has been associated with hepatitis B reactivation. We present a case where a patient, who developed bullous pemphigoid from immunotherapy, was started on rituximab without proper screening, after which his Hepatitis B core antibody came back positive. The patient was subsequently treated for Hepatitis B and completed a course of rituximab.
Case Description/
Methods: A 72-year-old male with history of hypertension, hyperlipidemia, and psoriasis presented with symptoms of worsening dysphagia to his primary care physician’s office. The patient underwent esophagogastroduodenoscopy with findings concerning for distal esophageal adenocarcinoma. He saw Oncology and was treated with radiation, a chemotherapy regimen of carboplatin and paclitaxel and surgical resection. He completed 5 rounds of chemotherapy and started immunotherapy with nivolumab, to be continued for 1 year. He developed a macular rash 7 months into immunotherapy. Thought to be related to his psoriasis, he was treated with topical corticosteroids. However, with no improvement in his symptoms he returned to Oncology and was treated with IVIG. He had mild resolution of his symptoms and was placed on a prednisone taper. He returned a few weeks later with his initial rash. He was referred to Dermatology and started on rituximab. He then had labs drawn with his Gastroenterologist and was positive for hepatitis b core antibody. With concern for Hepatitis B reactivation, he was started on tenofovir alafenamide for suppression. He completed treatment for his bullous pemphigoid and had his hepatic function monitored throughout therapy, which remained normal.
Discussion: This case illustrates a complex interplay between oncologic treatment, immunotherapy-related adverse events, and pre-existing conditions. The initiation of rituximab was aimed at controlling the bullous pemphigoid, which can be triggered by immune checkpoint inhibitors, such as nivolumab. The patient started rituximab without proper screening prior to initiation. This case highlights the importance of vigilance for thorough testing prior to initiating certain monoclonal antibodies, such as rituximab. The patient’s positive hepatitis B core antibody signaled past exposure, and rituximab heightened the risk for reactivation. This case emphasizes the significance of thorough screening, timely recognition, and appropriate prophylactic and therapeutic interventions to optimize patient outcomes.

Figure: This table depicts the patient's Hepatitis panel drawn following initiation of rituximab. The Hepatitis B core antibody is positive; however, the remainder of the testing is negative, indicating no acute infection.

Figure: This table depicts the serial monitoring of the patient's hepatic function while on rituximab and tenofovir alafenamide for adequate suppression therapy. His hepatic function remained normal throughout treatment.
Disclosures:
Kavina Munshi indicated no relevant financial relationships.
Huzaif Taufiq indicated no relevant financial relationships.
Tehilla Apfel indicated no relevant financial relationships.
Kavina Munshi, DO1, Huzaif Taufiq, MD2, Tehilla Apfel, MD3. P6099 - A Rash Decision: Navigating Immune-Related Dermatologic and Hepatic Complications in Esophageal Cancer Treatment, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
