Tuesday Poster Session
Category: Liver
P6020 - Delayed-Onset Drug-Induced Liver Injury and Immune Hemolytic Anemia: An Uncommon but Critical Complication
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Abdelhaleem Sideeg, MD
Virginia Mason Franciscan Health
Seattle, WA
Presenting Author(s)
Abdelhaleem Sideeg, MD1, Farah Saleem, MD1, Abdelaziz Mohamed, MBBS2, Fatima Elmustafa, MBBS3, Blaire Burman, MD1
1Virginia Mason Franciscan Health, Seattle, WA; 2One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 3Henry Ford Warren, Warren, MI
Introduction: Cefoxitin is commonly used as prophylaxis in bariatric surgery to prevent postoperative infections. Although drug-induced liver injury (DILI) and drug-induced immune hemolytic anemia (DIIHA) are uncommon, both are potentially life-threatening adverse drug reactions. Their concurrent occurrence following cefoxitin administration is rare. To our knowledge, this is the first reported case of simultaneous DILI and DIIHA associated with cefoxitin.
Case Description/
Methods: A 47-year-old woman with a history of HTN, GERD, and remote biopsy-proven DILI from ampicillin 24 years ago presented with fatigue, nausea, vomiting, and jaundice three weeks after undergoing Roux-en-Y gastric bypass surgery, during which she received cefoxitin. Vital signs: BP 115/72, HR 82, RR, 19. On physical exam, she had deep jaundice and mild epigastric tenderness. Lab results showed markedly elevated LFTs (ALT 1,026 U/L, AST 1,250 U/L, total bilirubin 53 mg/dL), anemia (hemoglobin dropped from 11.6 g/dL to 5.9 g/dL), LDH, and D-dimer; low haptoglobin; a positive direct antiglobulin test (DAT) for anti-IgG and polyspecific antibodies; and RBC agglutination resolving with warming. Abdominal and pelvic CT showed benign liver angiomyolipoma and mild biliary dilation. Liver biopsy showed eosinophilic portal infiltration, hepatocellular injury, and mild cholestasis consistent with DILI. There was no significant fibrosis, bile duct loss, or abnormal reticulin (Figure 1). Other etiologies, including viral and autoimmune hepatitis, were ruled out. The patient required six units of RBCs and was treated with methylprednisolone and rituximab. Upon discharge, she showed a significant improvement in hemoglobin levels and LFTs (table 1). The discharge plan included tapering prednisone and completing six months of rituximab therapy as an outpatient.
Discussion: DILI, often hypersensitivity-related, may occur after a single dose the offending drug within 1–3 weeks. DIIHA results from immune-mediated RBC destruction via drug-antibody interaction. DILI may be asymptomatic or present with jaundice, abdominal pain; DIIHA causes anemia symptoms such as fatigue and tachycardia. DILI diagnosis requires exclusion of other causes, timeline correlation, and sometimes biopsy. DIIHA is diagnosed by low hemoglobin and haptoglobin, elevated LDH, and positive DAT. Steroids treat severe DILI; DIIHA may require steroids and rituximab to prevent DIC.
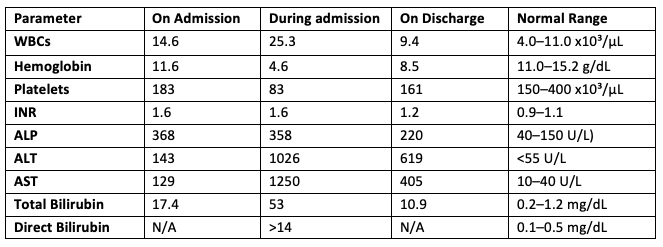
Figure: Table (1): Labs results
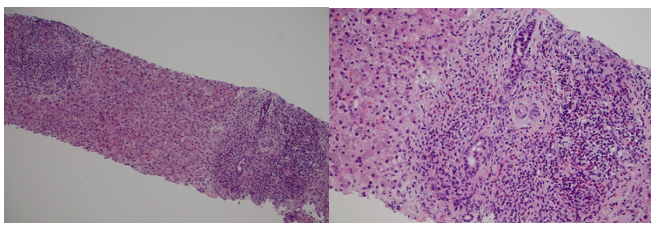
Figure: Figure (1): Liver Biopsy
Disclosures:
Abdelhaleem Sideeg indicated no relevant financial relationships.
Farah Saleem indicated no relevant financial relationships.
Abdelaziz Mohamed indicated no relevant financial relationships.
Fatima Elmustafa indicated no relevant financial relationships.
Blaire Burman indicated no relevant financial relationships.
Abdelhaleem Sideeg, MD1, Farah Saleem, MD1, Abdelaziz Mohamed, MBBS2, Fatima Elmustafa, MBBS3, Blaire Burman, MD1. P6020 - Delayed-Onset Drug-Induced Liver Injury and Immune Hemolytic Anemia: An Uncommon but Critical Complication, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Virginia Mason Franciscan Health, Seattle, WA; 2One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 3Henry Ford Warren, Warren, MI
Introduction: Cefoxitin is commonly used as prophylaxis in bariatric surgery to prevent postoperative infections. Although drug-induced liver injury (DILI) and drug-induced immune hemolytic anemia (DIIHA) are uncommon, both are potentially life-threatening adverse drug reactions. Their concurrent occurrence following cefoxitin administration is rare. To our knowledge, this is the first reported case of simultaneous DILI and DIIHA associated with cefoxitin.
Case Description/
Methods: A 47-year-old woman with a history of HTN, GERD, and remote biopsy-proven DILI from ampicillin 24 years ago presented with fatigue, nausea, vomiting, and jaundice three weeks after undergoing Roux-en-Y gastric bypass surgery, during which she received cefoxitin. Vital signs: BP 115/72, HR 82, RR, 19. On physical exam, she had deep jaundice and mild epigastric tenderness. Lab results showed markedly elevated LFTs (ALT 1,026 U/L, AST 1,250 U/L, total bilirubin 53 mg/dL), anemia (hemoglobin dropped from 11.6 g/dL to 5.9 g/dL), LDH, and D-dimer; low haptoglobin; a positive direct antiglobulin test (DAT) for anti-IgG and polyspecific antibodies; and RBC agglutination resolving with warming. Abdominal and pelvic CT showed benign liver angiomyolipoma and mild biliary dilation. Liver biopsy showed eosinophilic portal infiltration, hepatocellular injury, and mild cholestasis consistent with DILI. There was no significant fibrosis, bile duct loss, or abnormal reticulin (Figure 1). Other etiologies, including viral and autoimmune hepatitis, were ruled out. The patient required six units of RBCs and was treated with methylprednisolone and rituximab. Upon discharge, she showed a significant improvement in hemoglobin levels and LFTs (table 1). The discharge plan included tapering prednisone and completing six months of rituximab therapy as an outpatient.
Discussion: DILI, often hypersensitivity-related, may occur after a single dose the offending drug within 1–3 weeks. DIIHA results from immune-mediated RBC destruction via drug-antibody interaction. DILI may be asymptomatic or present with jaundice, abdominal pain; DIIHA causes anemia symptoms such as fatigue and tachycardia. DILI diagnosis requires exclusion of other causes, timeline correlation, and sometimes biopsy. DIIHA is diagnosed by low hemoglobin and haptoglobin, elevated LDH, and positive DAT. Steroids treat severe DILI; DIIHA may require steroids and rituximab to prevent DIC.

Figure: Table (1): Labs results

Figure: Figure (1): Liver Biopsy
Disclosures:
Abdelhaleem Sideeg indicated no relevant financial relationships.
Farah Saleem indicated no relevant financial relationships.
Abdelaziz Mohamed indicated no relevant financial relationships.
Fatima Elmustafa indicated no relevant financial relationships.
Blaire Burman indicated no relevant financial relationships.
Abdelhaleem Sideeg, MD1, Farah Saleem, MD1, Abdelaziz Mohamed, MBBS2, Fatima Elmustafa, MBBS3, Blaire Burman, MD1. P6020 - Delayed-Onset Drug-Induced Liver Injury and Immune Hemolytic Anemia: An Uncommon but Critical Complication, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
