Tuesday Poster Session
Category: Liver
P6010 - Famotidine-Induced Hepatocellular Injury in Pregnancy: A Diagnostic Challenge in a Patient With Ulcerative Colitis and Cholelithiasis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Rishika Trivedi, MD2, Mahmoud Barbarawi, MD2, Asif Zamir, MD, FACG3
1DHR Health, Edinburg, Tx, McAllen, TX; 2DHR Health, McAllen, TX; 3DHR Health Gastroenterology, Edinburg, TX
Introduction: Elevation of liver enzymes in pregnancy often prompts evaluation for viral hepatitis, autoimmune liver disease, or cholestasis. However, medication-induced hepatotoxicity remains under-recognized, particularly with widely used agents such as famotidine. We report a case of hepatocellular injury associated with famotidine use in a pregnant woman with ulcerative colitis (UC) and incidental cholelithiasis.
Case Description/
Methods: A 28-year-old woman with a history of UC (diagnosed 2015) presented at 27 weeks’ gestation with asymptomatic transaminitis. Laboratory workup showed ALT 481 U/L, AST 149 U/L, Alk Phos 135 U/L, and normal bilirubin. She was on stable mesalamine therapy with no UC flare since 2020. Ultrasound revealed hepatic steatosis and multiple mobile gallstones without cholecystitis. Autoimmune and viral hepatitis panels were negative. Notably, the patient had started famotidine (Pepcid) for reflux during pregnancy. Upon discontinuation of famotidine, her liver function tests improved significantly over two weeks without additional intervention. Liver enzymes continued to decline at one-month follow-up. Colonoscopy confirmed only mildly active chronic colitis, and no flare symptoms were noted during follow-up.
Discussion: Famotidine is commonly used in pregnancy for GERD and is generally considered safe. However, rare reports link it to idiosyncratic hepatocellular injury. In this case, the temporal relationship between famotidine use and abrupt LFT elevation, followed by resolution upon cessation, supports a diagnosis of famotidine-induced liver injury. The absence of symptomatic UC activity or biliary obstruction further supports drug-related hepatotoxicity. The case underscores the importance of comprehensive medication review when evaluating abnormal LFTs, even in patients with coexisting hepatobiliary conditions.
This case highlights a rare instance of famotidine-associated hepatocellular injury in pregnancy. Clinicians should maintain a high index of suspicion for medication-related liver enzyme abnormalities, even with widely used and presumed safe agents. Discontinuation of the offending agent can lead to rapid resolution and prevent unnecessary invasive diagnostics.
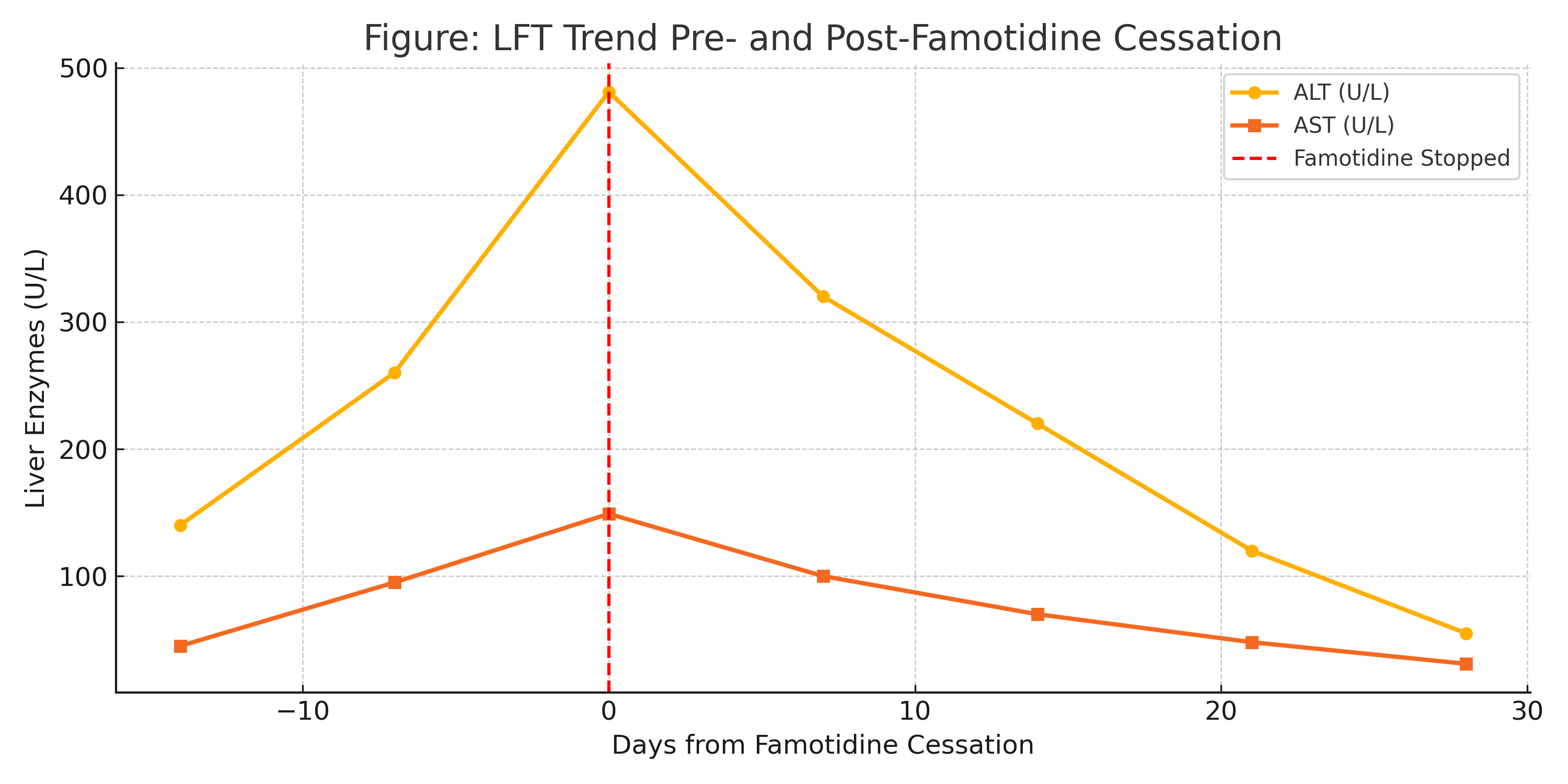
Figure: Figure: LFT trend graph pre- and post-famotidine cessation
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Mahmoud Barbarawi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Rishika Trivedi, MD2, Mahmoud Barbarawi, MD2, Asif Zamir, MD, FACG3. P6010 - Famotidine-Induced Hepatocellular Injury in Pregnancy: A Diagnostic Challenge in a Patient With Ulcerative Colitis and Cholelithiasis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2DHR Health, McAllen, TX; 3DHR Health Gastroenterology, Edinburg, TX
Introduction: Elevation of liver enzymes in pregnancy often prompts evaluation for viral hepatitis, autoimmune liver disease, or cholestasis. However, medication-induced hepatotoxicity remains under-recognized, particularly with widely used agents such as famotidine. We report a case of hepatocellular injury associated with famotidine use in a pregnant woman with ulcerative colitis (UC) and incidental cholelithiasis.
Case Description/
Methods: A 28-year-old woman with a history of UC (diagnosed 2015) presented at 27 weeks’ gestation with asymptomatic transaminitis. Laboratory workup showed ALT 481 U/L, AST 149 U/L, Alk Phos 135 U/L, and normal bilirubin. She was on stable mesalamine therapy with no UC flare since 2020. Ultrasound revealed hepatic steatosis and multiple mobile gallstones without cholecystitis. Autoimmune and viral hepatitis panels were negative. Notably, the patient had started famotidine (Pepcid) for reflux during pregnancy. Upon discontinuation of famotidine, her liver function tests improved significantly over two weeks without additional intervention. Liver enzymes continued to decline at one-month follow-up. Colonoscopy confirmed only mildly active chronic colitis, and no flare symptoms were noted during follow-up.
Discussion: Famotidine is commonly used in pregnancy for GERD and is generally considered safe. However, rare reports link it to idiosyncratic hepatocellular injury. In this case, the temporal relationship between famotidine use and abrupt LFT elevation, followed by resolution upon cessation, supports a diagnosis of famotidine-induced liver injury. The absence of symptomatic UC activity or biliary obstruction further supports drug-related hepatotoxicity. The case underscores the importance of comprehensive medication review when evaluating abnormal LFTs, even in patients with coexisting hepatobiliary conditions.
This case highlights a rare instance of famotidine-associated hepatocellular injury in pregnancy. Clinicians should maintain a high index of suspicion for medication-related liver enzyme abnormalities, even with widely used and presumed safe agents. Discontinuation of the offending agent can lead to rapid resolution and prevent unnecessary invasive diagnostics.

Figure: Figure: LFT trend graph pre- and post-famotidine cessation
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Mahmoud Barbarawi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Rishika Trivedi, MD2, Mahmoud Barbarawi, MD2, Asif Zamir, MD, FACG3. P6010 - Famotidine-Induced Hepatocellular Injury in Pregnancy: A Diagnostic Challenge in a Patient With Ulcerative Colitis and Cholelithiasis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
