Tuesday Poster Session
Category: Liver
P6008 - Nitrofurantoin-Induced Liver Injury in Pregnancy Mimicking Intrahepatic Cholestasis: A Mixed Hepatocellular-Cholestatic Pattern With Marked Bile Acid Elevation
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Rishika Trivedi, MD2, Mahmoud Barbarawi, MD2, Asif Zamir, MD, FACG3
1DHR Health, Edinburg, Tx, McAllen, TX; 2DHR Health, McAllen, TX; 3DHR Health Gastroenterology, Edinburg, TX
Introduction: Liver injury in pregnancy demands a careful diagnostic approach to avoid misclassification that could lead to premature delivery or unnecessary intervention. Intrahepatic cholestasis of pregnancy (ICP) and drug-induced liver injury (DILI) often share overlapping biochemical and clinical features. We present a case of nitrofurantoin-induced DILI mimicking ICP, with a mixed hepatocellular-cholestatic pattern.
Case Description/
Methods: A 22-year-old primigravida at 21 weeks' gestation presented with dark urine, scleral icterus, and mild pruritus, four days after completing a 5-day course of nitrofurantoin for urinary tract infection. Labs on admission revealed AST 1146 U/L, ALT 819 U/L, ALP 137 U/L, total bilirubin 7.7 mg/dL (direct 5.4), and serum bile acids 253.5 µmol/L. INR remained stable (peak 1.25). Extensive workup was negative for viral hepatitis, autoimmune markers, and metabolic disease. MRCP and ultrasound showed hepatic steatosis without biliary obstruction. No steroids or hepatoprotective therapy were initiated during hospitalization.
LFTs began declining spontaneously following nitrofurantoin discontinuation. Ursodiol 300 mg BID was started post-discharge. By 28 weeks, bilirubin normalized to 0.8 mg/dL, and transaminases returned to baseline. The patient remained asymptomatic without obstetric complications.
Discussion: Nitrofurantoin is frequently prescribed in pregnancy and generally well tolerated, yet idiosyncratic hepatotoxicity remains an underrecognized complication. This case exhibited a classic DILI trajectory: abrupt onset following drug exposure, mixed injury pattern, exclusion of alternative etiologies, and spontaneous resolution following withdrawal—prior to ursodiol initiation. The bile acid level ( >250 µmol/L) is among the highest reported in nitrofurantoin-related injury and may falsely suggest ICP, which more commonly presents in late pregnancy and with mild transaminitis.
In pregnant patients presenting with cholestatic features and significant transaminitis, DILI should be considered—even with medications deemed safe in pregnancy. This case highlights the importance of detailed medication history and temporal mapping to avoid misdiagnosis and unnecessary intervention.
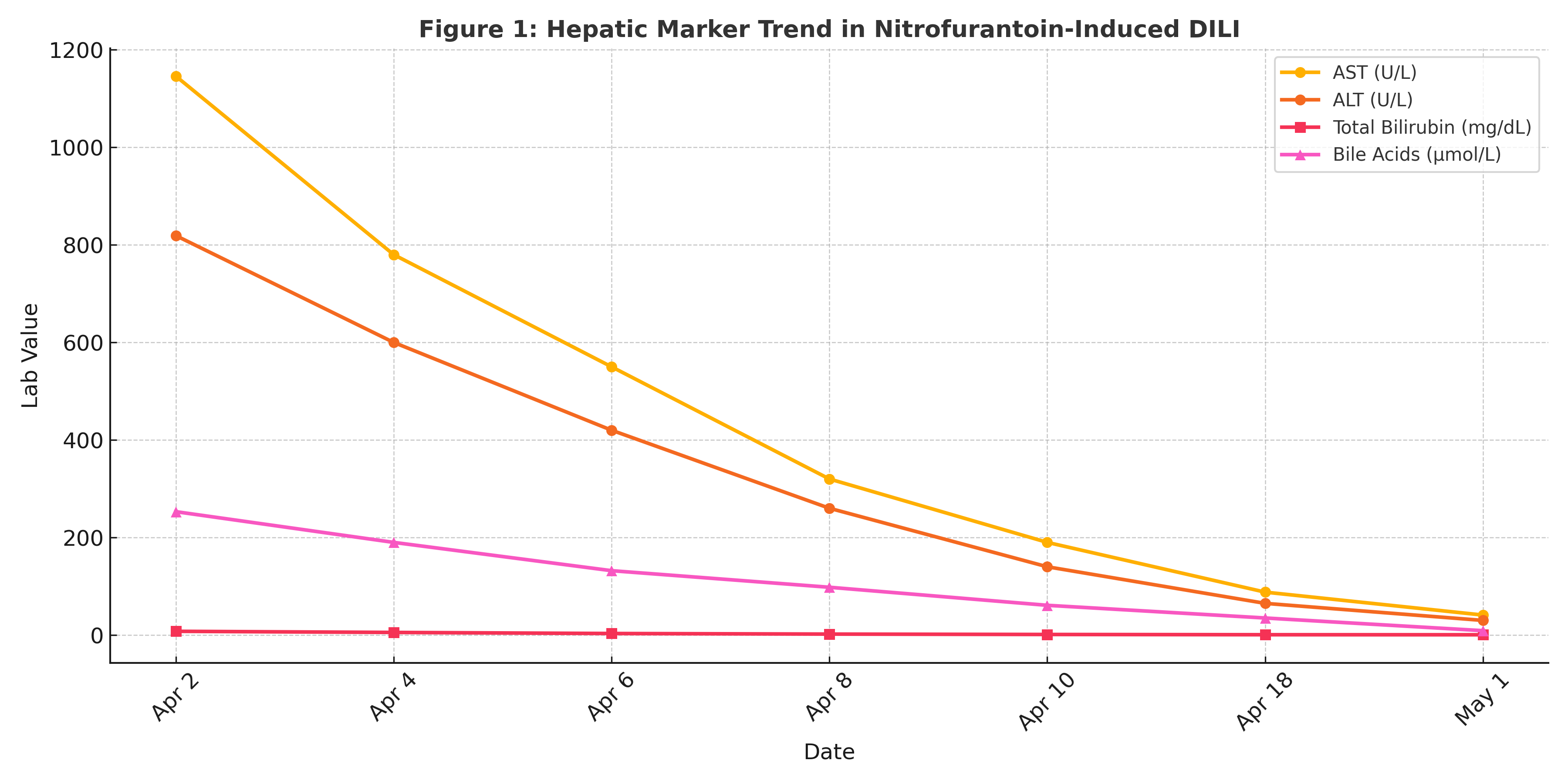
Figure: Figure 1. Trend of AST, ALT, total bilirubin, and bile acids in a pregnant patient with nitrofurantoin-induced liver injury. Liver enzyme normalization occurred prior to ursodiol initiation, supporting DILI as the primary etiology.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Mahmoud Barbarawi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Rishika Trivedi, MD2, Mahmoud Barbarawi, MD2, Asif Zamir, MD, FACG3. P6008 - Nitrofurantoin-Induced Liver Injury in Pregnancy Mimicking Intrahepatic Cholestasis: A Mixed Hepatocellular-Cholestatic Pattern With Marked Bile Acid Elevation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2DHR Health, McAllen, TX; 3DHR Health Gastroenterology, Edinburg, TX
Introduction: Liver injury in pregnancy demands a careful diagnostic approach to avoid misclassification that could lead to premature delivery or unnecessary intervention. Intrahepatic cholestasis of pregnancy (ICP) and drug-induced liver injury (DILI) often share overlapping biochemical and clinical features. We present a case of nitrofurantoin-induced DILI mimicking ICP, with a mixed hepatocellular-cholestatic pattern.
Case Description/
Methods: A 22-year-old primigravida at 21 weeks' gestation presented with dark urine, scleral icterus, and mild pruritus, four days after completing a 5-day course of nitrofurantoin for urinary tract infection. Labs on admission revealed AST 1146 U/L, ALT 819 U/L, ALP 137 U/L, total bilirubin 7.7 mg/dL (direct 5.4), and serum bile acids 253.5 µmol/L. INR remained stable (peak 1.25). Extensive workup was negative for viral hepatitis, autoimmune markers, and metabolic disease. MRCP and ultrasound showed hepatic steatosis without biliary obstruction. No steroids or hepatoprotective therapy were initiated during hospitalization.
LFTs began declining spontaneously following nitrofurantoin discontinuation. Ursodiol 300 mg BID was started post-discharge. By 28 weeks, bilirubin normalized to 0.8 mg/dL, and transaminases returned to baseline. The patient remained asymptomatic without obstetric complications.
Discussion: Nitrofurantoin is frequently prescribed in pregnancy and generally well tolerated, yet idiosyncratic hepatotoxicity remains an underrecognized complication. This case exhibited a classic DILI trajectory: abrupt onset following drug exposure, mixed injury pattern, exclusion of alternative etiologies, and spontaneous resolution following withdrawal—prior to ursodiol initiation. The bile acid level ( >250 µmol/L) is among the highest reported in nitrofurantoin-related injury and may falsely suggest ICP, which more commonly presents in late pregnancy and with mild transaminitis.
In pregnant patients presenting with cholestatic features and significant transaminitis, DILI should be considered—even with medications deemed safe in pregnancy. This case highlights the importance of detailed medication history and temporal mapping to avoid misdiagnosis and unnecessary intervention.

Figure: Figure 1. Trend of AST, ALT, total bilirubin, and bile acids in a pregnant patient with nitrofurantoin-induced liver injury. Liver enzyme normalization occurred prior to ursodiol initiation, supporting DILI as the primary etiology.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Rishika Trivedi indicated no relevant financial relationships.
Mahmoud Barbarawi indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Rishika Trivedi, MD2, Mahmoud Barbarawi, MD2, Asif Zamir, MD, FACG3. P6008 - Nitrofurantoin-Induced Liver Injury in Pregnancy Mimicking Intrahepatic Cholestasis: A Mixed Hepatocellular-Cholestatic Pattern With Marked Bile Acid Elevation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
